विद्या ददाति विनयं विनयाद्याति पात्रताम्। पात्रत्वाद्धनमाप्नोति धनाद्धर्मं ततः सुखम्॥
Knowledge brings humility; from humility comes worthiness; with worthiness comes wealth and with wealth one performs duties; in performing duties, one becomes happy.
Decoding the Preamble: The Soul of the Indian Constitution
We, the People of India…” These opening words are not...
Read Moreराज्य सभा क्या है – गठन, शक्तियां, सदस्य निर्वाचन 2025 | UPSC
राज्य सभा भारत की संसद का ऊपरी सदन है जो...
Read Moreसंविधान संशोधन (Amendment of the Constitution)
संविधान संशोधन क्या है? (Amendment of the Constitution) सामाजिक परिवर्तनों,...
Read Moreभारतीय संविधान में नागरिको के मूल कर्तव्य (Fundamental Duties)
नागरिको के मूल कर्तव्य सामान्य रूप से मूल कर्तव्य उन...
Read Moreराज्य की नीति के निदेशक तत्व (Directive Principles of State Policy)
सामान्य परिप्रेक्ष्य में, राज्य की नीति के निदेशक तत्वों को...
Read Moreसंस्कृति एवं शिक्षा से संबंधित अधिकार तथा संवैधानिक उपचार का अधिकार
अल्पसंख्यक समुदायों के हितों की सुरक्षा (Protection of Interests of...
Read Moreधर्म की स्वतंत्रता का अधिकार (Right to Freedom of Religion) (अनुच्छेद 25-28)
(Right to Freedom of Religion) (अनुच्छेद 25-28) अनुच्छेद 25(1) के...
Read Moreशोषण के विरुद्ध अधिकार (Right Against Exploitation) (अनुच्छेद 23-24)
शोषण के विरुद्ध अधिकार मानव दुर्व्यापार और अनैच्छिक श्रम का...
Read Moreस्वतंत्रता का अधिकार क्या है? (अनुच्छेद 19-22) (Right to Freedom)
स्वतंत्रता का अधिकार भारतीय संविधान में निहित एक मौलिक और...
Read MoreWhat Are Coral Reefs? Ecosystem Facts & Conservation 2025
Coral reefs are marine ecosystems formed by colonies of coral...
Read MoreHow Islands Form: The Geology Behind Hawaii, Madagascar, and Dubai
Imagine a landmass entirely surrounded by water, isolated from the...
Read MoreTypes of Mountains: Formation, Classification & Examples
Mountains are elevated landforms created through various geological processes over...
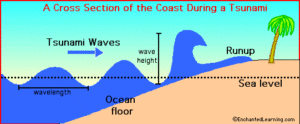
Read MoreWhat Causes a Tsunami? Complete Guide to Tsunami Formation, Warning Systems & Protection
Tsunami Tsunamis are primarily caused by underwater earthquakes that displace...
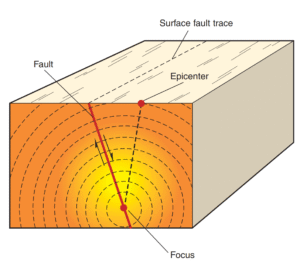
Read MoreComplete Guide to Earthquakes: Types, Causes & Effects 2025
Earthquakes are sudden releases of energy from Earth's crust that...
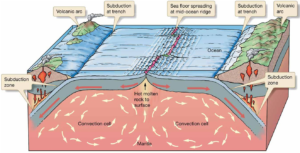
Read MoreThe Theory of Plate Tectonics
The theory of plate tectonics explains how Earth's outer shell...
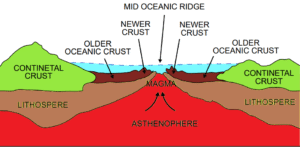
Read MoreWhat is Sea Floor Spreading? Complete Guide to Plate Tectonics Theory
Sea floor spreading is a geological process where new oceanic...
Read Moreप्रवाल भित्ति क्या है? प्रकार, वितरण और महत्व
प्रवाल भित्ति समुद्री प्रवाल कीटों द्वारा निर्मित कैल्शियम कार्बोनेट संरचनाएं...
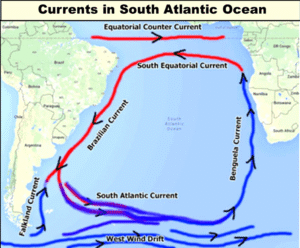
Read Moreमहासागरीय धाराएँ (Ocean Currents)
ज्वार-भाटा और तरंगों की भांति जलधाराओं से भी महासागरीय जल...
Read MoreGATE Geology 2022 Exam Solutions: A Complete Guide for Aspiring Geologists
Question Paper- Q.11 Which one of the following is the...
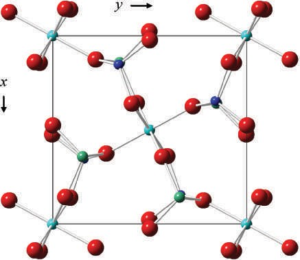
Read MoreGarnet Group Minerals: Composition, Structure, Properties, and Uses
Garnet Group The minerals of the garnet group crystallize in...
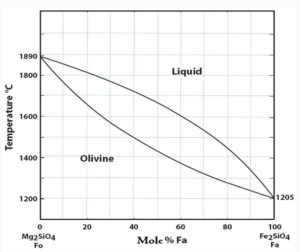
Read MoreOlivine Group: Structure, Properties, and Occurrence
Olivine Group Introduction General Formula: R2SiO4, where R = Mg,...
Read MoreSolution to GATE 2023 Geology GG1 Numerical Problem
The bulk density and water content of a soil are...
Read MoreGate Geology and Geophysics 2024 Question paper with solution
Question: 11 The Earth’s magnetic field originates from convection in...
Read MorePhotogeology in Modern Earth Science: Advantages, Limitations, and GIS Integration
Photogeology is the science and practice of interpreting aerial and...
Read MoreScope of Remote Sensing in Natural Resources Survey
Scope of Remote Sensing in Natural Resources Survey The wealth...
Read MoreIsotope Geothermometry: How Rocks Record Ancient Heat
How do we know the temperature of an ancient ocean...
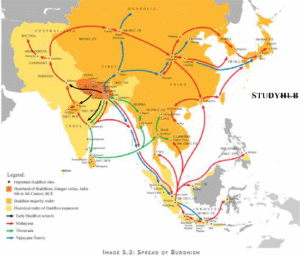
Read MoreBuddhism: Origins, Philosophy, and Traditions
Buddhism, originating in ancient India around the 6th-5th century BCE...
Read MoreGupta Art and Architecture – UPSC
Gupta Art and Architecture reached its zenith during the Gupta...
Read MorePost Mauryan Art and Architecture- UPSC
After the decline of the Mauryan Empire in 2nd century...
Read MoreMauryan Art and Architecture- UPSC
Mauryan Art and Architecture With the advent of Buddhism and...
Read MoreHarappan Art and Architecture- UPSC
A flourishing civilisation emerged on the banks of the river...
Read MoreThe History of Buddhism in india
Gautama Buddha (563 BC-483 BC) Doctrines of Buddha Mahatma Buddha...
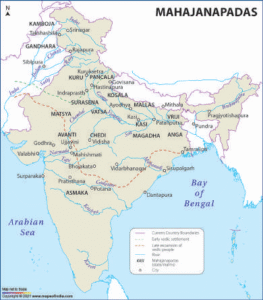
Read MoreThe Mahajanapadas: Ancient India’s Political Landscape
The period spanning roughly from the 6th to the 4th...
Read MoreRise of Mahajanapadas
Introduction India’s history is appropriately regarded as significant from the sixth...
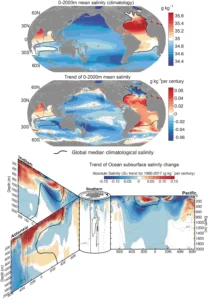
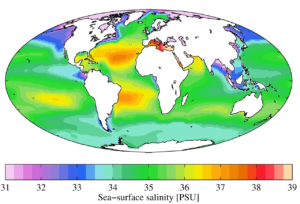
Read MoreOcean Salinity Variations: 5 Key Factors & Global Patterns
Ocean salinity variations result from five primary physical processes that...
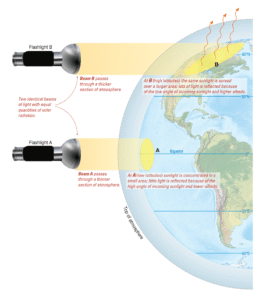
Read MoreHow does latitude affect solar radiation?
How Latitude Affects the Distribution of Solar Radiation If Earth...
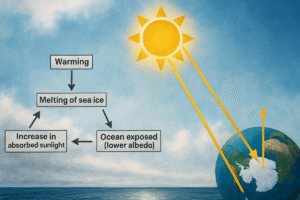
Read MoreWhat is the ocean heating phenomenon? Complete Guide
Ocean heating phenomenon refers to the systematic redistribution of thermal...
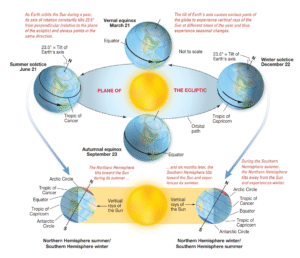
Read MoreWhy Do We have Seasons on Earth?
What Causes Earth’s Seasons? This seemingly straightforward inquiry often leads...
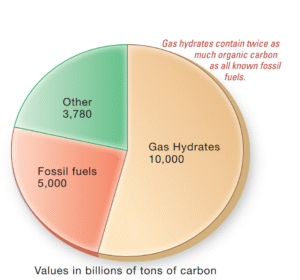
Read MoreWhat resources are derived from marine sediments?
What Resources Do Marine Sediments Provide? The ocean floor harbors...
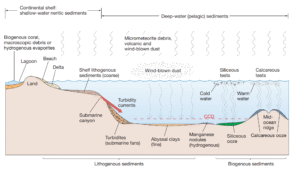
Read MoreHow Are Pelagic and Neritic Deposits Distributed?
How are Pelagic and Neritic Deposits Distributed in the Ocean?...
Read MoreWhat Are the Characteristics of Cosmogenous Sediment?
Cosmogenous sediment (cosmos = universe, generare = to produce) originates...
Read MoreWhat Are the Characteristics of Hydrogenous Sediment?
Hydrogenous sediment (from hydro, meaning water, and generare, meaning to...
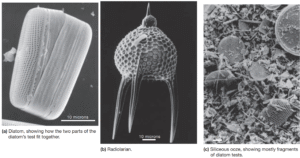
Read MoreWhat Are the Characteristics of Biogenous Sediment?
Biogenous sediment (from bio, meaning life, and generare, meaning to...
Read MoreStatic GK: StudyHUB
Indian constitution: StudyHUB
Geology: StudyHUB
Geography: StudyHUB
History: StudyHUB
Space Science: StudyHUB
StudyHUB – Free Open Educational Resources
Your complete guide for competitive exam preparation.
Welcome to StudyHUB
At StudyHUB, we strive to provide students and job aspirants with comprehensive study materials and tools to achieve their goals. Our resources cover a wide range of subjects, including History, Polity, Geography, MP GK, India GK, and much more. We also offer current affairs updates, quizzes, and valuable courses designed to cater to competitive exams at all levels.
We are now providing completely free PYQs and MCQs quizzes for all exams in both Hindi and English.
What is StudyHUB?
StudyHUB is a one-stop platform for aspirants preparing for competitive exams like:
- UPSC (Union Public Service Commission)
- MPPSC (Madhya Pradesh Public Service Commission)
- RPSC (Rajasthan Public Service Commission)
- UPPSC (Uttar Pradesh Public Service Commission)
- State PSC Exams across India
- Banking Exams (IBPS, SBI PO/Clerk, RBI Exams)
- GATE (Graduate Aptitude Test in Engineering)
- SSC Exams (Staff Selection Commission)
- Many more national and state-level examinations.
Why Choose StudyHUB?
Comprehensive Study Material
We provide high-quality study notes and PDFs for all subjects, tailored to specific exams.
Regular Current Affairs Updates
Stay updated with daily, weekly, and monthly current affairs, crucial for exams like UPSC, State PSC, and Banking.
Engaging Quizzes
Test your knowledge with topic-wise quizzes and full-length mock tests to enhance your preparation.
Job Notifications
Never miss an opportunity with our timely updates on government and private job openings.
Expert Tips and Guidance
Get valuable tips and strategies to crack exams from experienced mentors.
Our Features
- Subject-Wise Resources: Dedicated materials for History, Geography, Polity, MP GK, India GK, and more.
- Custom Quizzes: Practice with quizzes designed to match exam patterns.
- GATE Resources: Specially curated content for GATE aspirants.
- Exam Preparation Tips: Strategies to manage time, solve questions effectively, and improve accuracy.
- Job Alerts: Real-time updates on government and private job vacancies.
Study Tips for Aspirants
- Stay Organized: Create a timetable and stick to it.
- Revise Regularly: Go through notes and quizzes multiple times to retain information.
- Practice Mock Tests: Attempt mock tests to simulate the real exam experience.
- Stay Updated: Follow our current affairs section for the latest news and events.
- Ask for Help: Join our community to discuss doubts and seek guidance from experts.
How to Get Started?
- Explore our website for study materials, current affairs, and quizzes.
- Take quizzes and download PDFs to boost your preparation.
- Contact us for guidance and tips from our experienced mentors.
Contact Us
Have questions or need assistance? We’re here to help! Reach out to us anytime through our contact page on the website.