Olivine Group
Introduction
- Minerals of the Olivine Group include a number of closely related silicates which crystallize in the orthorhombic system.
- They belong to the Neso silicate group of silicates.
- High relief, high birefringence, and absence of cleavage are characteristic features.
General Formula: R2SiO4, where R = Mg, Fe, Mn, & Ca.
- Forsterite: Mg2SiO4
- Fayalite: Fe2SiO4
- Tephroite: Mn2SiO4
- Knebelite: (Mn, Fe)2SiO4
- Larnite: Ca2SiO4
- Monticellite: CaMgSiO4
- Kirschsteinite: CaFeSiO4
Structure
The structure consists of independent SiO4 tetrahedra linked by divalent (Mg, Fe) atoms in octahedral coordination (each of which has six nearest oxygen neighbors).
Octahedra in a structure are represented as (M): M1 < M2 < M3 < M4
Note: In olivine, only M1 & M2 sites are present. Fe and Mg will settle on either M1 or M2 site with no specific preference. If Ca is present, it will settle in the M2 site.
Phase Transformation
- At higher pressure, olivine adopts the denser structure of a spinel known as Wadsleyite and Ringwoodite.
- Olivine is abundant in the upper mantle and occurs as a perovskite structure at greater depth.
- At about 400 km depth, olivine is isochemically transformed into a denser structure with closer packing, called a spinel structure. At a depth of 440 km, it undergoes significant changes.
- At a depth of 660 km, further transformation occurs in olivine.
Note: In perovskite structure, a 6-oxygen cluster surrounds each silicon, and 8 such octahedra group around Mg.
Chemistry
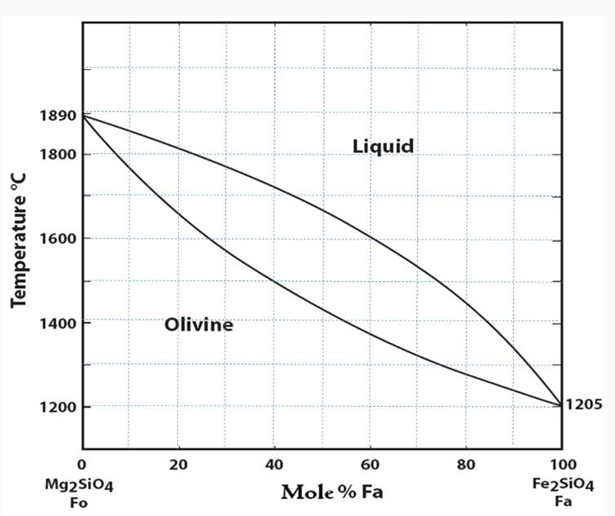
- The composition of olivine varies from Forsterite to Fayalite, and there is a complete solid solution between them. They constitute excellent examples of an isomorphous series and display solid solution behavior.
- Forsterite and Fayalite are names restricted to compositions Fo 100-90 and Fo 10-0, respectively.
- Forsterite (Fe 0-10%) or (Mg 100-90%)
- Chrysolite: Fe 20%
- Hyalosiderite: Fe 40%
- Hortonolite: Fe 60%
- Ferro hortonolite: Fe 80%
- Fayalite: Fe 90-100%
- Fayalite (Fe2SiO4), Knebelite (Mn,Fe)2SiO4, and Tephroite (Mn2SiO4) form a complete solid solution.
- Ni and Cr are commonly present in Mg-rich olivines as substituting elements.
- Al cannot be present in olivine composition due to charge differences.
- At very high pressure and temperature, olivine transforms into a spinel structure, which is an example of isomorphism.
- With Forsterite, quartz does not coexist, but with Fayalite, quartz exists.
- In the system MgO-SiO2, Forsterite and Enstatite are stable phases.
- Orthopyroxene reaction rims around olivine are seen in many gabbros and dolerites.
- Minerals that form solid solutions are called isomorphs. They have similar atomic structures and crystal morphology.
Physical Properties of Olivine
- Form: Prismatic crystals, massive and compact, granular grains
- Colour: Shades of green (olive green) to black
- Streak: Colourless
- Cleavage: Absent
- Lustre: Vitreous; transparent to translucent
- Fracture: Conchoidal
- Hardness (H): 6.5-7
- Specific Gravity (Sp. G): 3.2 (Forsterite) to 4.3 (Fayalite)
Optical Properties
- Optic sign: Forsterite is Biaxial (+Ve), and others are Biaxial (-Ve).
- Colour: Mg-rich olivine is colourless in thin sections, while Fe-rich olivine is pale yellow to green.
- Relief: High & (+Ve)
- Refractive Index: Increases from Forsterite to Fayalite.
Alteration
- Minerals of the olivine group are highly susceptible to temperature changes, oxidation, hydrothermal processes, low-grade metamorphism, and weathering. Alteration products are often fine-grained, e.g., iron oxides (hematite, magnetite), iddingsite, bowlingite, serpentine (antigorite, lizardite, or chrysotile).
- Serpentinization is the most important form of alteration and the most common process in metamorphism of olivine-rich rocks.
- The main alteration products of Mg-rich olivine are the 3-serpentine polymorphs: 1) Lizardite, 2) Chrysotile, 3) Antigorite.
- Olivine in metamorphosed basic igneous rocks commonly shows coronas, which are concentric rims consisting of pyroxene and amphibole.
- Serpentinization occurs during ocean floor metamorphism.
Paragenesis
- Igneous rocks: Dunite, peridotite, olivine gabbros, basalts, dolerites, kimberlites, lamproites, and lamprophyres.
- Metamorphic rocks: Formed due to de-dolomitisation of impure dolomite by metamorphism, resulting in Forsterite marbles.
- Ophicalcite: A variety of serpentine, which is a product of olivine.
- Olivine is also present in stony meteorites.
- Olivine is an extremely minor mineral in sediments due to its lesser stability.
- Forsterite melts at 1890°C and has a very high melting point, making it useful as a refractory material.
- Tephroite and Knebelite occur in Mn ores.
Varieties
Peridot: A gem variety of olivine.
Occurrence in India
- Ultramafic complexes at Baula Nuasahi, Orissa
- Kondapalle, Andhra Pradesh
- Salem, Tamil Nadu