Carbon Isotopes
Carbon has two stable isotopes with the following abundances:
- 12C: 98.89%
- 13C: 1.11%
Measurements are made relative to a standard belemnite sample known as PDB – Belemnitella americana from the Cretaceous Peedee formation, South Carolina. The original material of the PDB standard is now exhausted, and current standard materials are a carbonatite (NBS-18) and a marine limestone (NBS-19).
The ratio 13C/12C is measured in parts per thousand using the formula:
13C/12C (sample) - 13C/12C (standard) / 13C/12C (standard) × 1000
Carbon isotopes are measured as CO2 gas with a precision normally better than 0.1‰. The CO2 is liberated from carbonates with >1% phosphoric acid or by thermal decomposition. Organic compounds are oxidized at very high temperatures in a stream of oxygen or with an oxidizing agent such as CuO.
Distribution of Carbon Isotopes in Nature
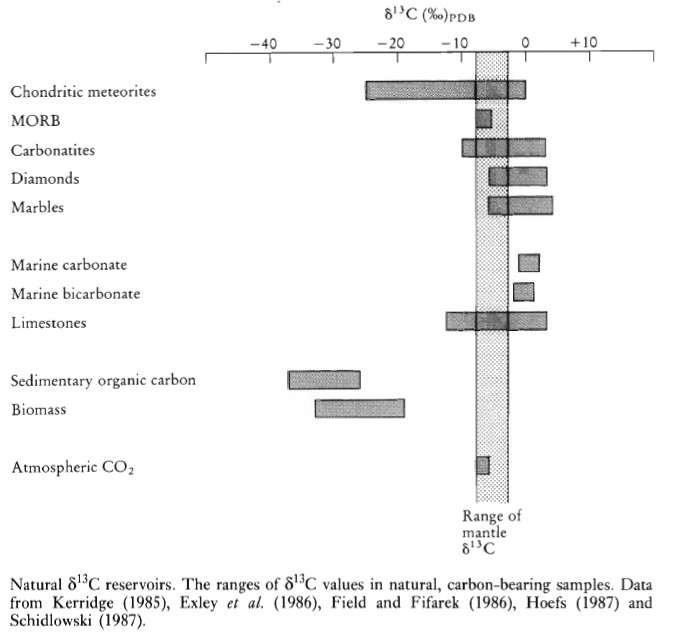
Carbon occurs in various forms in nature: oxidized (CO2, carbonates, and bicarbonates), reduced (methane and organic carbon), and as diamond and graphite. The range of carbon isotope compositions in natural substances is summarized as follows:
- Meteorites: Carbonaceous chondrites have bulk δ13C compositions in the range -25 to 0‰.
- Mantle: Values are in the narrow range δ13C = -3 to -8‰, with a mean value of about -6‰.
- MORB: δ13C = -6.6‰.
- Seawater: δ13C is close to 0‰.
- Marine Carbonate: Values range between -1 and +2‰.
- Marine Bicarbonate: Values range between -2 and 1‰.
Biologically derived (organic) carbon is isotopically light, i.e., depleted in δ13C. The conversion of inorganic carbon through a CO2 fixing mechanism into living organic carbon results in the preferential concentration of the light 12C isotope in organic carbon. Biological materials are strongly depleted in δ13C (-20 to -30‰; the mean value of terrestrial biomass is -26 ± 7‰). Methane is the most depleted of all carbon compounds and commonly has δ13C values of about -80‰.
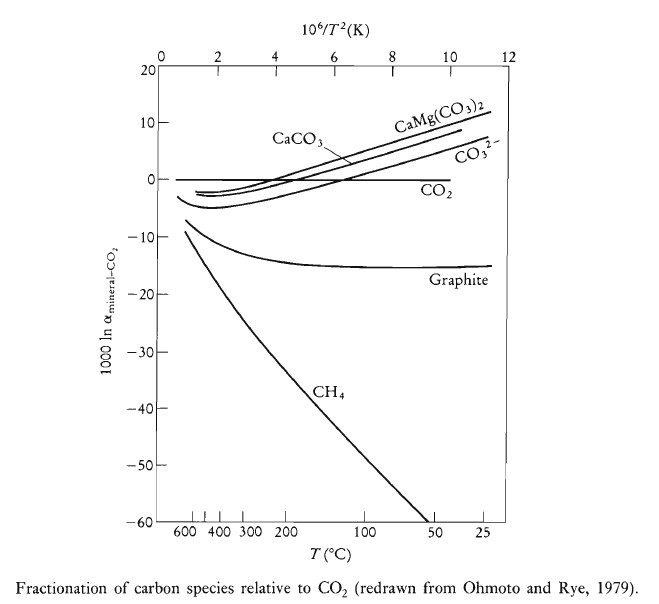
Controls on the Fractionation of Carbon Isotopes
The fractionation of carbon isotopes is controlled by both equilibrium and kinetic processes. Equilibrium fractionation is often temperature-dependent. Kinetic fractionation is important in biological systems, such as the fixing of CO2 as organic carbon and the evolution of methane from anaerobic fermentation. This type of fractionation is controlled by reaction rates and the greater readiness of the lighter isotope to react. When both carbon and oxygen are involved in the reaction, kinetic effects influence the isotopes of both elements similarly, leading to a correlation between δ18O and δ13C.
Combined Oxygen and Carbon Isotope Studies
Combined studies of carbon and oxygen isotopes in carbonates are useful for distinguishing between carbonates of different origins. The δ18O-δ13C plot helps in understanding limestone diagenesis. A study of carbon isotopes can determine the origin of carbon in the carbonate, distinguishing between marine, organic, and methane-related carbon. Oxygen isotope studies help determine the origin of fluids in equilibrium with carbonates and estimate the temperature of carbonate formation using the Epstein et al. (1953) thermometer. Fluid studies can calculate the composition of pore waters in equilibrium with calcite cements if the temperature of formation is known.
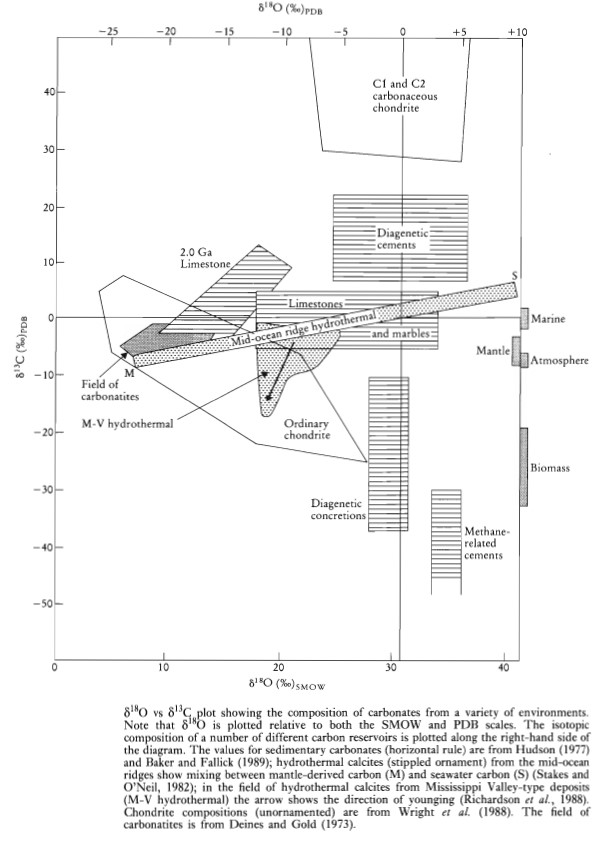
Hudson (1977) suggests that different crystalline forms of carbonate have grown at different times, and analyzing these forms isotopically allows constructing an evolutionary pathway on a δ18O-δ13C diagram. A typical limestone isotopic history involves various crystalline generations, with recent advances like the laser microprobe offering higher spatial resolution.
Hydrothermal Calcite
Hydrothermal calcites formed by water-rock interaction at mid-ocean ridges show a wide range of compositions on a δ18O-δ13C plot, reflecting varying water/rock ratios. High-temperature calcites in greenstone breccia have mantle-like δ13C values, while low-temperature vein calcites have seawater δ13C values. Mississippi Valley-type lead-zinc mineralization calcites in Carboniferous limestone show a marked decrease in δ13C, indicating that early calcites were similar to limestone carbonates, while late-stage fluids involved degradation of organic carbon.
Seawater δ13C Values
Seawater has a δ13C value close to 0‰, with marine carbonate values between -1 and +2‰. Ancient seawater has varied, with significant excursions noted in the Phanerozoic record. For instance, excursions of about 6‰ PDB in the Permian and 4.8‰ in the Cretaceous serve as stratigraphic markers. Precise records for the Tertiary are used for stratigraphic correlation. Anomalous δ13C values can indicate local conditions or global anoxic events with increased deposition of light organic carbon into black shales and corresponding increases in δ13C in seawater bicarbonate.
Biogeochemical Evolution
Carbon isotope studies in sedimentary carbon – carbonate and organic carbon – trace ancient biological activity through the geological record. Schidlowski (1987, 1988) demonstrated that isotopically light organic carbon and isotopically heavy carbonates are complementary reservoirs, responding to changes in biological activity. The stability of biological activity since 3.8 Ga is evidenced by minimal change in δ13C values in carbonates and organic carbon throughout geological history.
Carbon Isotopes in CO2
CO2 in igneous, metamorphic, and mineralizing fluids can be traced to its source using carbon isotopes. Studies focus on CO2 from fluid inclusions and carbonate minerals in equilibrium with CO2-rich fluids. However, fractionation processes complicate the determination of CO2 origins. Examples include:
- CO2 Dissolved in Igneous Melts: CO2 exsolved from igneous melts can be traced to mantle CO2. Step-heating experiments reveal two fractions of CO2 with different δ13C values, suggesting either organic contamination or residue remaining after CO2 fractionation and outgassing.
- CO2 in Metamorphic Fluids: Decarbonation of marine limestone during metamorphism leads to lower δ13C values in calcite and CO2 enriched in δ13C. Metamorphism of biogenic carbon results in higher δ13C values in residual carbon or graphite.
- CO2 Fluid-Rock Interaction: Fluid-rock interaction affects δ13C values depending on the fluid/rock ratio. The shift in δ13C from the original value is used to calculate the extent of fluid-rock interaction.