Overview of Earth’s Atmosphere
The universe contains billions of galaxies and each galaxy is made up of billions of stars. Stars are hot glowing balls of gas that generate energy by converting hydrogen into helium near their centers. Our sun is an average-sized star situated near the edge of the Milky Way galaxy. Revolving around the sun are Earth and seven other planets. Our solar system comprises these planets, along with a host of other material (comets, asteroids, meteors, dwarf planets, etc.).
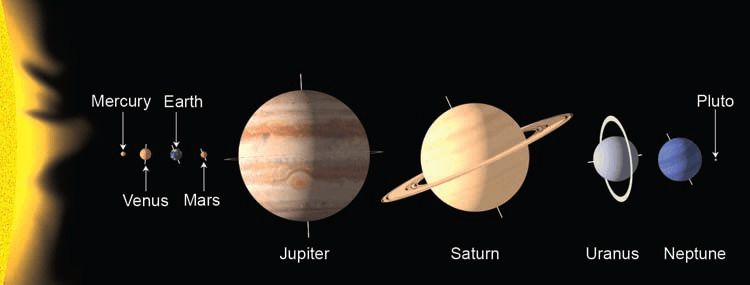
Warmth for the planets is provided primarily by the sun’s energy. At an average distance from the sun of nearly 150 million kilometers (km) or 93 million miles (mi), Earth intercepts only a very small fraction of the sun’s total energy output.
Radiation is energy transferred in the form of waves that have electrical and magnetic properties. The light that we see is radiation, as is ultraviolet light.
Radiant energy (or radiation) that drives the atmosphere into the patterns of everyday wind and weather and allows Earth to maintain an average surface temperature of about 150C (590F).
This temperature is mild, Earth experiences a wide range of temperatures, as readings can drop below -850C (-1210F) during a frigid Antarctic night and climb, during the day, to above 500C (1220 F) on the oppressively hot subtropical desert.
Earth’s atmosphere is a relatively thin, gaseous envelope that comprises mostly nitrogen and oxygen, with small amounts of other gases, such as water vapor and carbon dioxide (CO2)
Our atmosphere extends upward for many hundreds of kilometers, it gets progressively thinner with altitude. Almost 99 percent of the atmosphere lies within a mere 30 km (19 mi) of Earth’s surface.
THE EARLY ATMOSPHERE
The atmosphere that originally surrounded Earth was probably much different from the air we breathe today. Earth’s first atmosphere (some 4.6 billion years ago) was most likely hydrogen and helium—the two most abundant gases found in the universe—as well as hydrogen com pounds, such as methane (CH4) and ammonia (NH 3).
Millions of years passed, the constant outpouring of gases from the hot interior—known as outgassing—provided a rich supply of water vapor, which formed into clouds.
COMPOSITION OF TODAY’S ATMOSPHERE
The various gases present in a volume of air near Earth’s surface. Nitrogen (N2) occupies about 78 percent and molecular oxygen (O2) about 21 percent of the total volume of dry air. These percentages for nitrogen and oxygen hold fairly constant up to an elevation of about 80 km (50 mi).
Composition of the Atmosphere near the Earth’s Surface
Permanent Gases
| Gas | Symbol | Percent (by Volume) Dry Air |
|---|---|---|
| Nitrogen | N₂ | 78.08 |
| Oxygen | O₂ | 20.95 |
| Argon | Ar | 0.93 |
| Neon | Ne | 0.0018 |
| Helium | He | 0.0005 |
| Hydrogen | H₂ | 0.00006 |
| Xenon | Xe | 0.000009 |
Variable Gases
| Gas (and Particles) | Symbol | Percent (by Volume) | Parts per Million (ppm) |
|---|---|---|---|
| Water vapor | H₂O | 0 to 4 | – |
| Carbon dioxide | CO₂ | 0.041 | 410* |
| Methane | CH₄ | 0.00018 | 1.8 |
| Nitrous oxide | N₂O | 0.00003 | 0.3 |
| Ozone | O₃ | 0.000004 | 0.04** |
| Particles (dust, soot, etc.) | – | 0.00001 | 0.01–0.15 |
| Chlorofluorocarbons (CFCs) and | – | 0.0000001 | 0.0001 |
| hydrofluorocarbons (HFCs) |
Notes:
- For CO₂, 410 parts per million means that out of every million air molecules, 410 are CO₂ molecules.
- Stratospheric values for ozone at altitudes between 11 km and 50 km are about 5 to 12 ppm
At the surface, there is a balance between destruction (output) and production (input) of these gases.
Nitrogen is removed from the atmosphere primarily by biological processes that involve soil bacteria. Nitrogen is also taken from the air by tiny ocean-dwelling plankton that convert it into nutrients that help fortify the ocean’s food chain. It is returned to the atmosphere mainly through the decaying of plant and animal matter.
Oxygen, on the other hand, is removed from the atmosphere when organic matter decays and when oxygen combines with other substances, producing oxides. It is also taken from the atmosphere during breathing, as the lungs take in oxygen and release carbon dioxide (CO2). The addition of oxygen to the atmosphere occurs during photosynthesis.
The concentration of the invisible gas water vapor (H2O), however, varies greatly from place to place, and from time to time. Close to the surface in warm, steamy, tropical locations, water vapor may account for up to 4 percent of the atmospheric gases, whereas in colder arctic areas, its concentration may dwindle to a mere fraction of a percent. Water vapor molecules are, of course, invisible. They become visible only when they transform into larger liquid or solid particles, such as cloud droplets and ice crystals, which may grow in size and eventually fall to Earth as rain or snow.
The changing of water vapor into liquid water is called condensation.
The process of liquid water becoming water vapor is called evaporation.
The falling rain and snow is called precipitation.
In the lower atmosphere, water is everywhere. It is the only substance that exists as a gas, a liquid, and a solid at those temperatures and pressures normally found (N2) occupies about 78 percent and molecular oxygen (O2) about 21 percent of the total volume of dry air. If all the other gases are removed, these percentages for nitrogen and oxygen hold fairly constant up to an elevation of about 80 km (50 mi). Water vapor is an extremely important gas in our atmosphere. Water is the only substance that exist as a gas, a liquid, a solid at a temperature and pressure normally found near the earth’s surface. During transformation from gaseous stage to solid/liquid, water vapor releases a large amount of heat- called latent heat.
Latent heat is an important source of atmospheric energy, especially for thunderstorms and hurricanes. Water vapor is a potential greenhouse gas that absorbs the earth’s outgoing radiant energy.
Carbon dioxide is a natural component of the atmosphere occupying a small 0.04% of the volume of air. Carbon dioxide enters into the atmosphere mainly from decay of organic matter, volcanic eruption, exhalation of animals, burning of fossil fuels and deforestation.
The removal of CO2 from the atmosphere takes place during photosynthesis, as plants consume CO2 to produce green matter. The CO2 is then stored in roots, branches, and leaves. Rain and snow can react with silicate minerals in rocks and remove CO2 from the atmosphere through a process known as chemical weathering. The oceans act as a huge reservoir for CO2, as phytoplankton (tiny drifting plants) in surface water fix CO2 into organic tissues. Ocean contains 50 times more CO2 than the atmosphere.
Presently CO2 levels increasing at a rate of 1.5ppm/Yr. Like water vapor, CO2 is another greenhouse gas by absorbing portion of earth’s radiant energy. So, everything being equal, the increase in the atmospheric CO2 concentration will result in increase in average global average surface temperature.
Carbon dioxide and water vapor are not the only greenhouse gases. Others include methane (CH4), nitrous oxide (NO2), and chlorofluorocarbons (CFCs).
Methane appears to derive from the breakdown of plant material by certain bacteria in rice paddies, wet oxygen poor soil, the biological activity of termites, and biochemical reactions in the stomachs of cows, although some methane is also leaked into the atmosphere by natural-gas operations.
Chlorofluorocarbons (CFCs) represent a group of greenhouse gases.
Ozone (O3) is the primary ingredient of photochemical smog, which irritates the eyes and throat and damages vegetation. Atmospheric ozone (about 97 percent) is found in the stratosphere, where it is formed naturally, as oxygen atoms combine with oxygen molecules. The concentration of ozone averages less than 0.002 percent by volume but this small quantity is important, because it shields plants, animals, and humans from the sun’s harmful ultraviolet rays.
Tiny solid or liquid particles of various composition, suspended in the air, are called aerosols. Some natural impurities found in the atmosphere are quite beneficial. Some natural impurities let the water vapor to condense on them thereby forming the cloud. Most-human made impurities create health-hazard called pollutants. Ex- carbon monoxide, nitrogen dioxide, hydrocarbons. Burning of sulfur-containing fuels release colorless sulfur dioxide into the air. This sulfur dioxide transforms into sulfuric acids when combines with water vapor producing acid rain.
Reference:
Ahrens, C. Donald, and Robert Henson. Meteorology Today: An Introduction to Weather, Climate, and the Environment. 12th ed., Cengage Learning, 2018