Biogeochemical cycles are natural pathways through which essential elements like carbon, nitrogen, phosphorus, and sulfur circulate between living organisms and the environment. These cycles ensure continuous nutrient availability, supporting ecosystem stability, biodiversity, and all life processes on Earth.
What is the biogeochemical chemical cycle?
The living systems are fundamentally reliant on the transfer of energy and the circulation of nutrients occurring within ecosystems. Both elements profoundly determine the abundance of life forms, the rate of metabolism at which they sustain their existence, and the overall complexity inherent to the ecosystem structure. While energy traverses through ecosystems facilitating various biological processes, it is eventually dissipated as heat energy, becoming permanently unavailable for further utilization within the system. In contrast, the nutrient components of organic matter are never depleted, as they undergo continuous recycling in perpetuity.
For instance, during respiration, an individual might inhale millions of atoms that were previously inhaled by ancestral populations or other organisms, exemplifying the recyclable nature of these elements.
Carbon, hydrogen, oxygen, nitrogen, and phosphorus, in both elemental and compound states, collectively constitute approximately 97% of the human body’s total mass, and account for over 95% of the total biomass across all living organisms.
Beyond these, an additional 15 to 25 elements are essential in various forms to ensure the survival and optimal health of both flora and fauna.
These elements, commonly referred to as mineral nutrients, remain in a state of constant circulation, transitioning from abiotic to biotic systems and subsequently returning to the non-living environment, thereby maintaining a near-continuous looped exchange.
This cyclic movement of nutrients, spanning biological and geophysical realms, is referred to as the biogeochemical cycle—where “bio” denotes living systems and “geo” signifies the atmospheric and terrestrial domains.
What is meant by Nutrient Cycling?
- The concept of the nutrient cycle refers to the dynamic process through which nutrients transfer from the physical environment into living organisms, and are then returned to their original environmental reservoirs through natural recycling mechanisms. This bidirectional movement—from abiotic sources to plants and animals, and subsequently back to the environment—is crucial for sustaining life, serving as a fundamental ecological function within any given region.
- To ensure the long-term sustainability of organisms within a particular ecosystem, it is essential that the nutrient cycle remains balanced and stable over time.
- Nutrient cycling is generally examined with respect to individual nutrients, each of which exhibits a distinctive pattern of circulation within a specific environment.
- Among the most significant nutrient cycles are the carbon cycle and the nitrogen cycle, both of which form an essential component of the broader soil nutrient cycle.
- In addition to these, there exists a multitude of other nutrient cycles that hold notable ecological importance, encompassing a wide array of trace mineral nutrient cycles critical for ecosystem functioning.
Types of Nutrient Cycle
- Nutrient cycles are classified as either perfect or imperfect based on the duration required for nutrient replacement.
- A perfect nutrient cycle is characterized by the rapid replenishment of nutrients at the same rate they are consumed. Most gaseous cycles fall under this category due to their efficient recycling mechanisms.
- Conversely, sedimentary cycles are deemed relatively imperfect, as certain nutrients become permanently sequestered within sediments, rendering them unavailable for immediate reuse within the cycle.
- Classification can also be made based on the type of reservoir, resulting in two major categories: the gaseous cycle and the sedimentary cycle.
- Gaseous Cycle – in this type, the primary reservoir is located within the atmosphere or the hydrosphere.
- Sedimentary Cycle – here, the reservoir exists within the lithosphere, specifically the earth’s crust.
Gaseous Cycles
An examination of key gaseous cycles begins with three of the most crucial—the water, carbon, and nitrogen cycles.
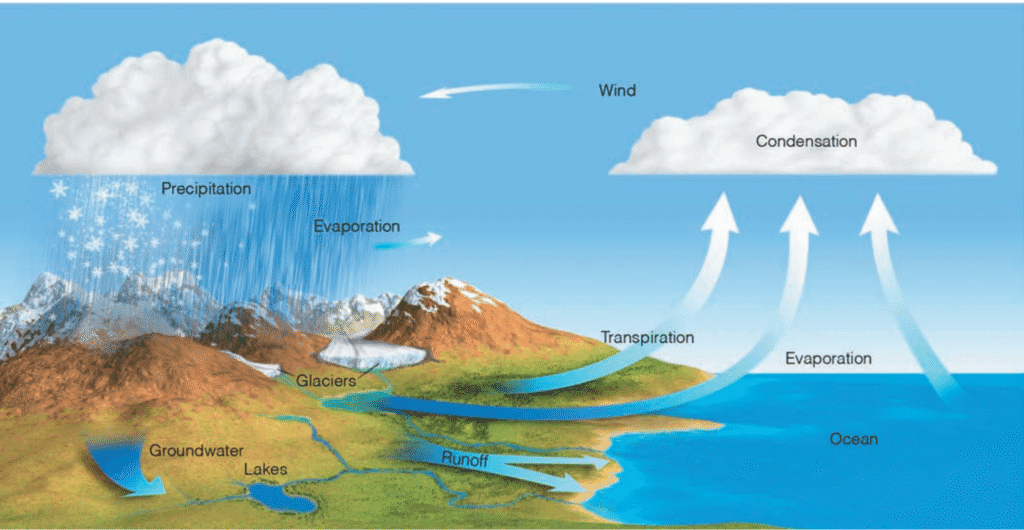
(a) Water Cycle (Hydrologic)
- Water serves as a fundamental ecological component, shaping both the structure and functionality of ecosystems. The circulation of all other nutrients is inherently linked to water, as it facilitates their transportation throughout various ecological processes.
- Functioning as a universal solvent, water enables the absorption of nutrients by living organisms.
- The hydrologic cycle represents the uninterrupted movement of water within the Earth-atmosphere system, primarily driven by solar radiation.
- Water is stored within major reservoirs, including the atmosphere, oceans, lakes, rivers, soil layers, glaciers, snowfields, and groundwater aquifers. Its transition between these reservoirs is governed by processes such as evaporation, transpiration, condensation, precipitation, deposition, runoff, infiltration, and groundwater flow.
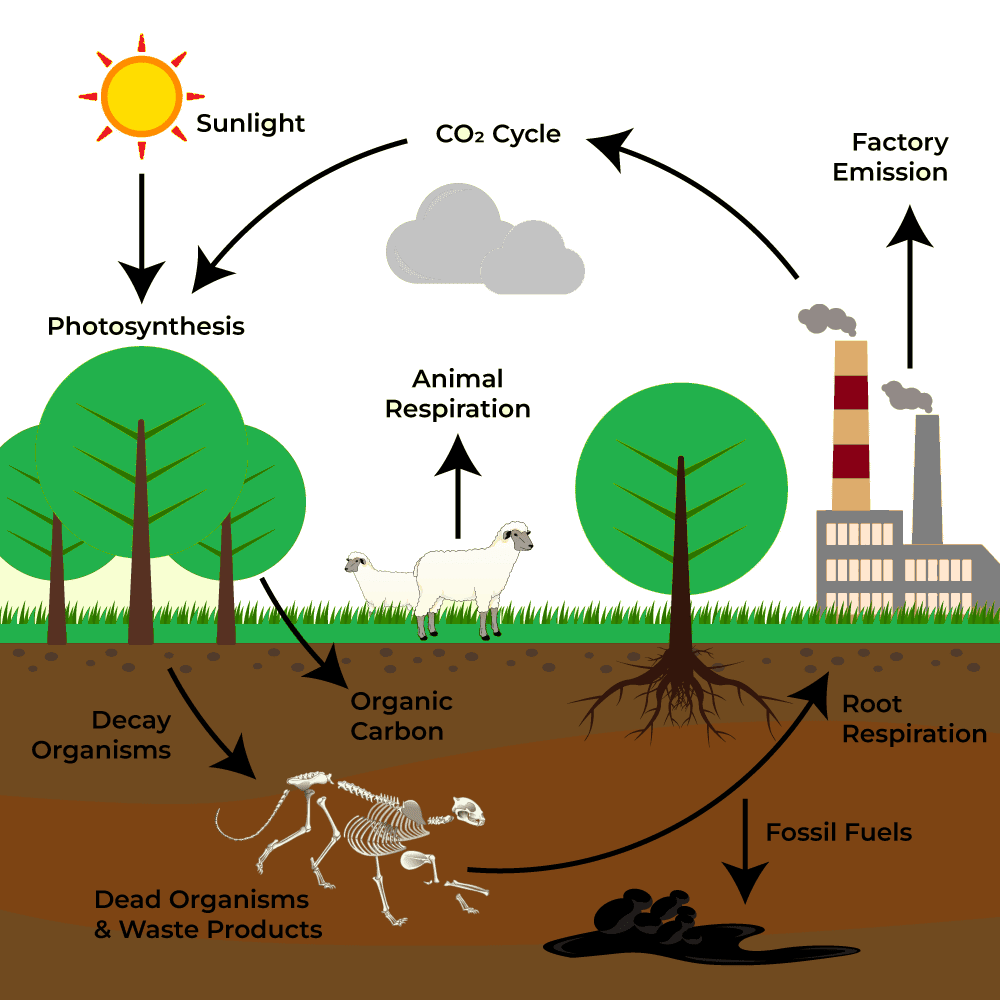
(b) What is in the carbon cycle?
- Though carbon constitutes a relatively minor component of the atmosphere when compared to oxygen and nitrogen, the presence of carbon dioxide is essential for life, as it plays a crucial role in the formation of carbohydrates through the process of photosynthesis in plants.
- Carbon is the primary element embedded in all organic compounds, ranging from fossil fuels like coal and oil to DNA (deoxyribonucleic acid), the molecular foundation of genetic inheritance.
- In the atmosphere, carbon predominantly exists as carbon dioxide (CO₂). The carbon cycle entails a constant exchange of carbon between the biosphere and the atmosphere.
- Through photosynthesis, atmospheric carbon enters green plants and is then transferred to animals through the food chain. It is subsequently returned to the atmosphere via respiration and the decomposition of organic remains. This represents a short-term cycle in which carbon flow remains dynamic and continuous.
- A portion of carbon also enters a long-term cycle, where it becomes stored as undecomposed organic material within the peat-rich layers of marshy soils or as insoluble carbonates deposited in the sediments of aquatic ecosystems, requiring extended geological periods for eventual release.
- In the deep oceanic zones, such carbon may remain buried for millions of years, until tectonic activity elevates these sedimentary rocks above sea level. Once exposed to surface erosion, these formations release carbon dioxide, carbonates, and bicarbonates into rivers and streams, thereby re-entering the active carbon exchange system.
- Fossil fuels such as coal, oil, and natural gas are organic substances that were buried underground prior to undergoing decomposition, and over time were converted into fossil energy sources through geological transformations.
- Upon combustion, the carbon content sequestered within these fuels is released back into the atmosphere in the form of carbon dioxide (CO₂), thereby reactivating it within the carbon cycle.
(c) The Nitrogen Cycle
Nitrogen is a vital component of proteins and serves as a fundamental structural element of all living tissues. It comprises approximately 16% of the total mass of proteins.
Although the atmosphere contains an unlimited reservoir of nitrogen, most living organisms are unable to utilize it in its elemental form (N₂). For biological assimilation, nitrogen must undergo a process called fixation, wherein it is transformed into ammonia, nitrites, or nitrates, which can then be absorbed by plants.
Nitrogen fixation on Earth occurs through three distinct mechanisms:
- Through the action of microorganisms such as nitrogen-fixing bacteria and cyanobacteria (blue-green algae)
- Via industrial methods, particularly through fertilizer manufacturing processes
- To a lesser degree, by natural atmospheric phenomena, including lightning and thunderstorms
The quantity of nitrogen fixed by humans through industrial activities has significantly surpassed the amount naturally fixed within the biogeochemical cycle. This has led to an excess accumulation of nitrogen, rendering it a pollutant capable of disturbing the ecological balance. Its overabundance can result in acid rain, eutrophication, and the proliferation of harmful algal blooms, all of which have serious environmental consequences.
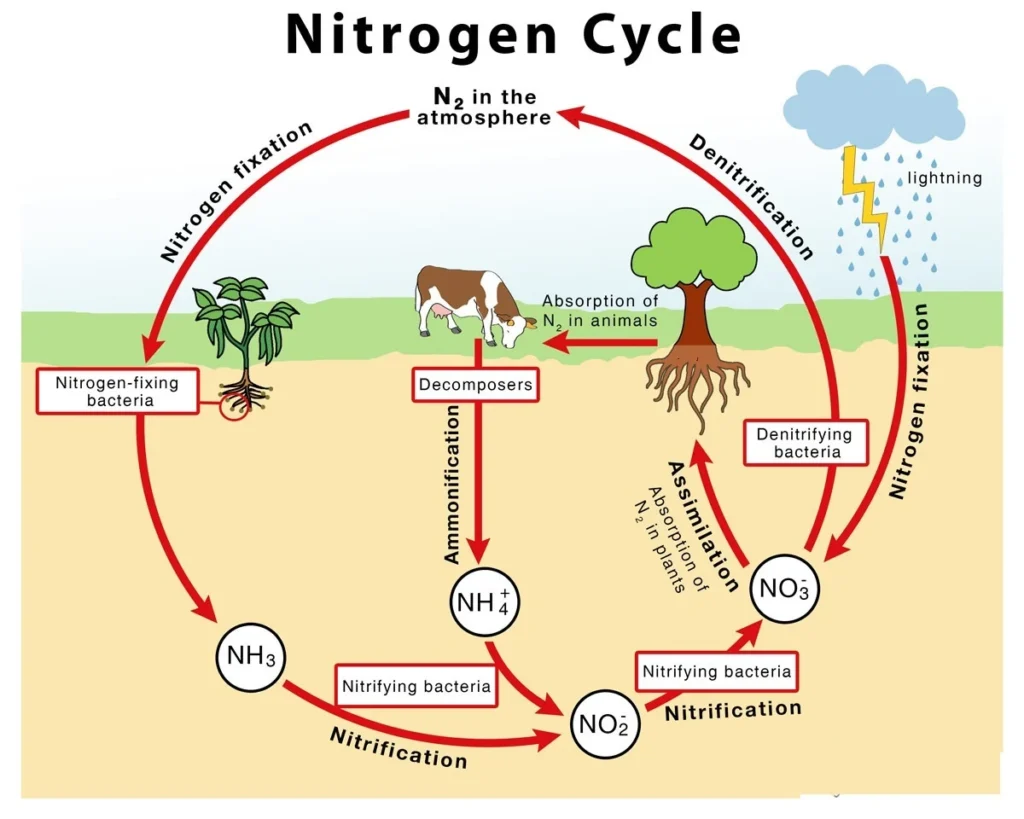
Several microorganisms possess the ability to convert atmospheric nitrogen into ammonium ions. These include free-living nitrifying bacteria, such as the aerobic Azotobacter and anaerobic Clostridium, along with symbiotic nitrifying bacteria associated with leguminous plant roots. In addition, certain non-leguminous plants also form symbiotic relationships with nitrogen-fixing bacteria like Rhizobium. Furthermore, cyanobacteria (blue-green algae) such as Anabaena and Spirulina contribute to biological nitrogen fixation.
Ammonium ions may be directly absorbed by certain plant species as a source of nitrogen, or they may undergo oxidation into nitrites and subsequently into nitrates through the activity of two specialized groups of nitrifying bacteria.
Nitrosomonas bacteria facilitate the conversion of ammonia into nitrite, which is then further oxidized into nitrate by Nitrobacter bacteria.
These nitrates, formed within the soil, are assimilated by plants and transformed into amino acids, the essential components of proteins, which then enter higher trophic levels within the ecosystem.
Following excretion or death, nitrogen is recycled back into the soil as ammonia.
A portion of soil nitrates, due to their high solubility, may be lost from the ecosystem via surface runoff or groundwater leaching.
Additionally, denitrifying bacteria such as Pseudomonas, present in both terrestrial and marine environments, convert nitrates and nitrites back into elemental nitrogen, which is then released into the atmosphere, thereby completing the nitrogen cycle.
Periodic thunderstorms also contribute by converting atmospheric nitrogen into ammonia and nitrates, which return to the soil through precipitation, becoming available once again to plants.
Sedimentary Cycle
- Phosphorus, calcium, and magnesium are among the key elements that undergo circulation through the sedimentary cycle.
- Unlike gaseous cycles, the elements involved in this process typically do not pass through the atmosphere, but instead follow a distinctive flow pattern encompassing erosion, sedimentation, orogenic (mountain-building) processes, volcanic activity, and biological transport, including dispersal via the excreta of marine birds.
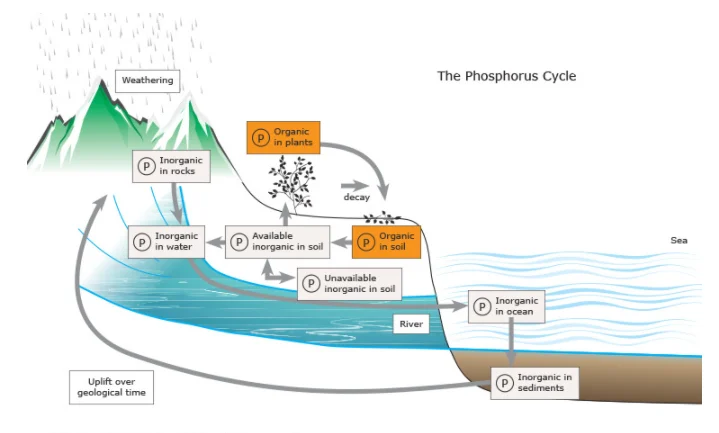
(a) Phosphorus Cycle
- Phosphorus serves a critical function within aquatic ecosystems, significantly influencing water quality. In contrast to carbon and nitrogen, which are primarily atmospheric in origin, phosphorus is found in substantial quantities within phosphate-rich mineral deposits and enters the biogeochemical cycle predominantly through erosion and mining operations.
- This nutrient is recognized as the primary contributor to the excessive proliferation of both rooted aquatic vegetation and free-floating microscopic algae in lake environments.
- The primary reservoir of phosphorus resides within the earth’s crust.
- On terrestrial surfaces, phosphorus predominantly exists in the form of phosphate compounds. Through weathering and erosional processes, these phosphates are transported into rivers and streams, eventually making their way to the oceanic systems.
- Within the marine environment, phosphorus gradually accumulates along continental shelves as insoluble deposits. Over geological time scales, tectonic uplift raises these sedimentary layers above sea level, exposing phosphate-rich formations on land.
- Subsequent weathering of these rocks releases phosphates, thereby initiating the geochemical phase of the phosphorus cycle once again.
(b) Sulphur Cycle
- The main reservoir of sulphur is located within soil and sedimentary layers, where it is stored in both organic forms—such as coal, oil, and peat—and inorganic forms, including pyrite and sulphur-bearing rocks, as sulphates, sulphides, and organic sulphur compounds.
- Sulphur is released into ecosystems through the weathering of rocks, erosional runoff, and the decomposition of organic materials, and is transported to terrestrial and aquatic systems in the form of dissolved salts.
- While the sulphur cycle is primarily sedimentary, it includes a gaseous component due to the presence of two volatile compounds: hydrogen sulphide (H₂S) and sulphur dioxide (SO₂), which contribute to atmospheric interactions within the cycle.
- Sulphur enters the atmosphere through a variety of natural and anthropogenic sources, including volcanic eruptions, the combustion of fossil fuels, ocean surface emissions, and gaseous byproducts from the decomposition of organic matter.
- Within the atmosphere, hydrogen sulphide (H₂S) undergoes oxidation, forming sulphur dioxide (SO₂). This sulphur dioxide is eventually absorbed by rainwater, returning to the Earth’s surface as diluted sulphuric acid.
- Regardless of its origin, sulphur, once present as sulphates, is absorbed by plants and integrated into their tissues via metabolic pathways, forming sulphur-containing amino acids, which become components of autotrophic proteins. This sulphur is then transferred through the grazing food chain.
- Sulphur bound within living organisms returns to the soil, as well as to the beds of aquatic ecosystems such as ponds, lakes, and oceans, through the excretion and decomposition of organic remains.