An aluminosilicate mineral is a type of mineral that contains both aluminum and silicon in its chemical composition.
Sillimanite(Al2SiO5)(Orthorhombic)
Sillimanite is a high-temperature polymorph of Al2SiO5 and occurs as colourless or white acicular to fibrous crystals in high grade metamorphosed pelitic rocks. It has high relief and straight extinction.
| a | 1.653—1.661 |
| b | 1.657—1.662 |
| g | 1.672—1.683 |
| d | 0.018—0.022 |
| 2Vg | 21—30° |
| Orientation | a = x, b = y, g = z; O.A.P. (010) Bxa \(001\) |
| D (g/cm³) | 3.23—3.27 |
| H | 6Ý—7Ý |
| Cleavage | {010} good, uneven transverse fractures |
| Colour | Normally colourless or white, also yellow, brown, greyish green, bluish green; colourless in thin section |
| Pleochroism | In thick sections, coloured varieties may be pleochroic with a pale brown or pale yellow, b brown or greenish, g dark brown or blue |
| Unit cell | a 7.48 Å, b 7.67 Å, c 5.77 Å; V 331 ų; Z = 4; space group Pbnm |
Structure
Sillimanite has a crystal structure composed of chains of edge-sharing aluminium-oxygen octahedra that run parallel to the z-axis. The lateral connection between these octahedral chains is formed by a double chain consisting of alternating silicon (Si) and aluminium (Al) in tetrahedral coordination. In sillimanite, aluminium exists in two different coordination states: half of the aluminium atoms are in octahedral coordination with an Al—O bond distance of 1.91 Å, while the other half are in tetrahedral coordination with an Al—O bond distance of 1.77 Å.

One key feature is that the Si—O tetrahedra do not share oxygens with other Si—O tetrahedra. The arrangement of octahedral chains in the three polymorphs of Al₂SiO₅ is similar across the minerals.

Chemistry
Sillimanite’s composition is relatively stable and close to the formula Al₂SiO₅. The most frequent ion that substitutes for aluminium in its structure is Fe³⁺. The small quantities of other elements that are sometimes reported likely represent impurities. Additionally, small amounts of absorbed and entrapped water are frequently found in the fibrous mass of crystals, particularly in the fibrolite variety of sillimanite.
Sillimanite can be synthesized from its constituent oxides under conditions of high temperature and pressure. The phase diagram for Al₂SiO₅ shows that the triple point between the polymorphs andalusite, sillimanite, and kyanite occurs at approximately 530 ± 20°C and 0.42 ± 0.03 GPa. When heated above about 1000°C, sillimanite becomes unstable and alters into mullite and quartz. Other alteration products include muscovite, sericite, pyrophyllite, kaolinite, and montmorillonite. Under conditions of stress or increasing pressure, sillimanite can convert into kyanite, though the reverse transformation to andalusite during cooling is slow.

Optical and Physical Properties
Sillimanite typically forms as long prismatic crystals or fibrous mats of fine crystalline material, commonly referred to as fibrolite. It can also occur as more equidimensional crystals. Its relief is moderately high, and its refractive indices show only slight variations. The birefringence is relatively large, though finely fibrous material may not always show the normal retardation effect. The {010} cleavage is often difficult to observe in thin sections. In cross-sections showing the dominant {110} form, the extinction is symmetrical with respect to the crystal boundaries.

Distinguishing Features
Sillimanite can be distinguished from andalusite by its positive (length-slow) elongation and higher birefringence. In comparison, apatite is length-fast and has weaker birefringence, while kyanite has higher refractive indices and a larger optic axial angle (2V). Differentiating between sillimanite and mullite can be challenging; this distinction often requires techniques such as single-crystal X-ray diffraction (XRD), infrared absorption spectroscopy, or electron probe microanalysis (EPMA).
Paragenesis
Sillimanite is the high-temperature polymorph of Al₂SiO₅. It is found in the higher grades of thermally metamorphosed argillaceous rocks, such as sillimanite-cordierite gneiss and biotite-sillimanite hornfels, where it often forms through the breakdown of biotite or earlier-formed andalusite. Sillimanite also occurs in the highest-grade regional metamorphic rocks, including micaceous sillimanite schist and coarser quartz-sillimanite gneiss.
Much of the sillimanite produced during regional metamorphism forms through the breakdown of muscovite and biotite, though it can also result from reactions between staurolite and quartz. The polymorphic transformation from kyanite to sillimanite tends to be sluggish, which can result in the persistence of kyanite within the sillimanite zone of metamorphism. In cases where regional metamorphism of pelitic rocks is followed by thermal metamorphism, sillimanite and andalusite may occur together as oriented intergrowths, as seen in sillimanite gneiss enclosed within the Ross of Mull granite.
Mullite and its Relation to Sillimanite
Mullite, a high-temperature aluminosilicate, shares many properties with sillimanite and is best distinguished using techniques such as single-crystal XRD, infrared absorption spectroscopy, or EPMA. Compositionally, mullite has less silica than sillimanite. Mullite is relatively rare in nature and typically occurs in pelitic xenoliths (buchites) found in basic igneous rocks. The type occurrence of mullite is on the Isle of Mull, where it is found in a fused xenolith of Jurassic shale in the tholeiitic portion of a composite sill. Iron-bearing mullite can be found in thermally metamorphosed lateritic rocks. Mullite is also a common refractory phase in ceramic products.
Andalusite (Al2SiO5)(Orthorhombic)
Andalusite, the low-temperature, low-pressure polymorph of Al2SiO5, is commonly found associated with cordierite in argillaceous rocks in contact aureoles. It has high relief and low birefringence. Included carbonaceous material may produce the variety chiastolite.
| a | 1.633—1.642a |
| b | 1.639—1.644 |
| g | 1.644—1.650 |
| d | 0.009—0.012 |
| 2Va | 73—86° |
| D (g/cm³) | 3.14—3.16a |
| H | 6Ý—7Ý |
| Orientation | a = z, b = y, g = x; O.A.P. (010) |
| Cleavage | {110} good, {100} poor; (110): (11 0) = 89° |
| Colour | Usually pink, but may be white or rose-red; also grey, violet, yellow, green or clouded with inclusions; in thin section normally colourless, but may be pink or green |
| Pleochroism | In coloured varieties weak, with a rose-pink, b and g greenish yellow |
| Twinning | Rare on {101} |
| Unit cell | a 7.79 Å, b 7.90 Å, c 5.55 Å; V 342 ų; Z = 4; space group Pnnm |

Structure
Andalusite has chains of edge-sharing distorted Al—O octahedra aligned parallel to the z-axis. These chains are linked laterally by chains of Si—O tetrahedra, which alternate with five-coordinated distorted Al—O trigonal bipyramids. The Si—O tetrahedra do not share oxygens with other Si—O tetrahedra. Among the three polymorphs, andalusite has the largest specific volume, with a volume of 17.1 ų per oxygen ion. This larger volume makes it the favored polymorph under low-pressure conditions.
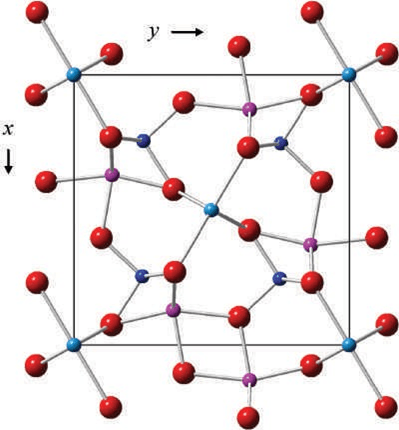
Chemistry
The composition of andalusite is typically close to Al2SiO5. The primary substituents reported in significant quantities are ferric iron and manganese. The substitution of Al by Fe3+ is generally minimal, with Fe2O3 content in most andalusites being less than 2%. The manganese-rich variety of andalusite, known as viridine, contains appreciable amounts of ferric iron and manganese. Viridine has been documented with up to 4.8% of Fe2O3 and 19.6% of Mn2O3. A phase containing 32.2% Mn2O3 has been described, leading to the chemical formula (Mn3+,Al)AlSiO5. The ideal end-member composition Mn3+AlSiO5 is referred to as kanonaite. Andalusite can be synthesized from kaolinite or from Al2O3 + SiO2 at temperatures between 450–650ºC and at water-vapor pressures ranging from 0.06 to 0.2 GPa. The phase diagram for the Al2SiO5 composition shows that the triple point between andalusite, sillimanite, and kyanite occurs at approximately 530±20ºC and 0.42±0.03 GPa. Andalusite can undergo alteration into sericite, with the variety chiastolite being especially susceptible to alteration along the lines of carbonaceous inclusions.

Optical and Physical Properties
The entry of ferric iron and manganese into the structure of andalusite increases both the refractive indices and density. The color and pleochroism of the mineral are primarily influenced by the Fe and Mn content. Pink and red varieties typically contain Fe, while the green varieties are rich in Mn. In the green variety viridine, the optic sign becomes positive, and pleochroism may range from yellow to emerald green. The variety chiastolite contains a regular arrangement of carbonaceous impurities, often forming a cruciform pattern when viewed in cross-section. These impurities are concentrated at the center of each crystal, corresponding to the initial stage of growth, and along the diagonals, representing the trace of the prism edges as the crystal grew. Foreign matter is brushed to the edges of the crystal during growth, which is most effective in directions perpendicular to the prism faces. When heated to temperatures between 1450 and 1500ºC, andalusite converts to mullite, a reaction utilized in the manufacture of refractories.

Distinguishing Features
Andalusite can be identified by its almost-square cross-section, high relief, low birefringence, and length-fast prismatic crystals. The pleochroic varieties of andalusite can be distinguished from orthopyroxenes by their length-slow character and the higher birefringence of orthopyroxenes.
Paragenesis
Andalusite is typically found in metamorphosed pelitic rocks surrounding igneous intrusions and is commonly associated with cordierite. In the early stages of metamorphism, it occurs as anhedral grains but later develops a prismatic outline, pushing aside enclosed foreign matter to form the chiastolite pattern. As metamorphism advances, andalusite becomes free of inclusions. Under conditions of higher temperature and pressure, andalusite may become unstable and alter into its polymorphs, sillimanite or kyanite. Andalusite and cordierite schists are occasionally found in regionally metamorphosed areas, such as the Banff area of northeast Scotland, where there appears to have been a reduction or relaxation in shearing stress. Andalusite is rarely found in granites, likely formed due to contamination. However, pseudomorphs of andalusite in quartz veins within pegmatites suggest that it may also have a pegmatitic or hydrothermal origin. The manganian andalusite variety, viridine, typically occurs in low-grade, regionally metamorphosed Mn-rich pelitic rocks, where it may be associated with piemontite, spessartine, chlorite, or hematite. Andalusite is also found as a detrital mineral in some sandstones.
Kyanite(Al2SiO5)(Triclinic)
Kyanite is the high-pressure polymorph of Al2SiO5 and occurs mainly in regionally metamorphosed pelitic rocks. Pale blue or white in hand specimen and colourless with high relief in thin section, it shows good cleavage and variable extinction angle depending on orientation.

| a | 1.710—1.718 |
| b | 1.719—1.724 |
| g | 1.724—1.734 |
| d | 0.012—0.016 |
| 2Va | 78—83° |
| Orientation | g^z on (100) = 27—32°, on (010) = 5—8°; a^z on (001) = 0—3°; Bxa nearly \(100\) |
| D (g/cm³) | 3.53—3.65 |
| H | 5Ý—7, variable |
| Cleavage | {100} perfect, {010} good, {001} parting; (001):z = 85° |
| Twinning | Lamellar on (100), twin axis \\(100) or || y or z; multiple on {001} |
| Colour | Blue to white, also grey, green, yellow, pink or black; colourless to pale blue in thin section |
| Pleochroism | Weak; in thick sections a colourless, b violet-blue, g cobalt-blue |
| Unit cell | a 7.12 Å, b 7.85 Å, c 5.57 Å; a 89.98°, b 101.12°, g 106.01°; V 293 ų; Z = 4; space group P1 |
Structure
Kyanite has a structure in which the oxygen atoms are arranged in a slightly distorted close-packed cubic array. Similar to andalusite and sillimanite, kyanite contains chains of Al—O octahedra that are linked together by Si, Al, and O atoms. Silicon is coordinated by four oxygen atoms, while aluminum is coordinated by six. The Si—O tetrahedra do not share corners with other Si—O tetrahedra, although due to projection, they may appear to overlap. The unit cell volumes of the three Al2SiO5 polymorphs confirm that kyanite has the lowest specific volume, making it the polymorph favored under the highest pressure conditions.
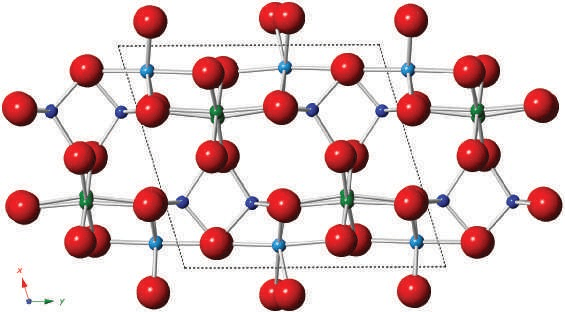
Chemistry
Kyanite, like other aluminosilicates, closely approximates Al2SiO5 with only limited amounts of Fe3+ able to enter the structure. Chromium (Cr) can also be present in moderate amounts. Small quantities of titanium (Ti) reported in kyanite are likely due to rutile inclusions, which are commonly found in the mineral. Recent studies show that in pure kyanite, the alkali content never exceeds 0.06%. Kyanite can be synthesized at temperatures of 900ºC and pressures between 1–4 GPa. The phase diagram for Al2SiO5 shows the triple point between kyanite, andalusite, and sillimanite to be approximately 530±20ºC and 0.42±0.03 GPa. When heated to around 1300ºC, kyanite converts to mullite and a glass. Alteration products include pyrophyllite, muscovite, and sericite. Kyanite may invert to sillimanite or andalusite with changes in pressure–temperature conditions, such as during regional metamorphism, where it converts to andalusite in the aureole of late granite. Calcined kyanite is used in refractory products and is the most economically important of the aluminosilicates due to its occurrence in large workable deposits.

Optical and Physical Properties
Kyanite exhibits high relief, especially for a mineral that is normally colorless in thin section. Its birefringence is moderate, producing higher first-order colors for sections of normal thickness. The optic axial plane is almost perpendicular to (100) and inclined at approximately 30º to (010) and the z-axis. The extinction position nearest to z corresponds to the slow ray, with the extinction angle varying from around 30º to zero, with basal sections having almost zero extinction. The color of kyanite varies from colorless to blue, often unevenly distributed. Hardness also varies depending on crystallographic direction and face.

Distinguishing Features
Kyanite can be identified by its high relief, which is higher than that of the other aluminosilicates. Its birefringence is less than that of sillimanite but greater than that of andalusite, and kyanite differs from andalusite in being length-slow. The distinctive maximum extinction angle of approximately 30º is observed in sections that give a negative biaxial figure with a large 2V. In detrital grains, kyanite may be recognized by its step-like features due to its good cleavage.
Paragenesis
Kyanite is typically found in regionally metamorphosed pelitic rocks, although it can also occur in psammites. It serves as a zonal mineral in pelitic assemblages, developing after staurolite and before sillimanite as metamorphism progresses. High-pressure kyanite-talc-quartz whiteschists are increasingly being recognized, such as in the Dora Maira assemblages in the Western Alps and the coesite-bearing whiteschists of Dabie Shan, China, where pressure–temperature estimates range from 3.7 to 3.4 GPa at 700–750ºC. Kyanite can also be derived from pyrophyllite or by the dehydration of paragonite in the presence of quartz. It may also invert from andalusite in regions where regional metamorphism is superimposed on thermal metamorphism. In some instances, kyanite occurs in thermal aureoles along with staurolite, possibly due to shear during the emplacement of the igneous body. Kyanite is also found in eclogites and kyanite amphibolites and has been reported in pegmatitic veins, although these are more likely quartz-kyanite segregation veins. Kyanite is also fairly common as a detrital mineral in sedimentary rocks.
Abbreviations and Symbols
| Abbreviation/Symbol | Description |
|---|---|
| A˚ (= 10 nm) | Ångström units (10–8 cm) |
| a | Cell edge in the x direction |
| a | Activity (of substance indicated by subscript) |
| atom % | Atoms per cent, percentage on an atomic basis |
| b | Cell edge in the y direction |
| Bxa | Acute bisectrix |
| c | Cell edge in the z direction |
| calc. | Calculated |
| D | Density (g/cm3) |
| d | Interplanar spacing |
| DTA | Differential thermal analysis |
| Eh | Redox potential |
| EPMA | Electron probe microanalysis |
| f | Fugacity (of substance indicated by subscript) |
| Fe* | Total Fe2+ + Fe3+ |
| H | Hardness (Mohs’ scale) |
| H2O— | Absorbed water |
| H2O+ | Water derived from mineral breakdown |
| hex (subscript) | Hexagonal |
| IR | Infrared spectroscopy |
| KD | Distribution coefficient |
| LA-ICP-MS | Laser ablation inductively coupled plasma mass spectrometry |
| M | mol/litre |
| M | Generalized cation site |
| Ma | Million years |
| meq., meq. | Milliequivalents, microequivalents |
| mol% | Molecules per cent, percentage on a molecular basis |
| n | Refractive index (for a cubic mineral) |
| nm | Nanometre (10–9 m) |
| NMR | Nuclear magnetic resonance |
| O.A.P. | Optic axial plane |
| P | Pressure (see note below on units of pressure) |
| pfu | Per formula unit |
| pH | —log (H+ concentration) |
| ppm | Parts per million |
| % | Parts per mille (parts per thousand) |
| R | Generalized symbol for group of metal ions |
| R | Reflectance |
| RE, REE | Rare earth, rare earth element |
| r < v (or r > v) | Optic axial angle in red light is less than (or greater than) that in violet light |
| rh (subscript) | Rhombohedral |
| SEM | Scanning electron microscopy |
| SIMS | Secondary ion mass spectrometry |
| TEM | Transmission electron microscopy |
| HRTEM | High resolution transmission electron microscopy |
| T | Temperature. Also ‘tetrahedral site’ |
| tr. | Trace |
| VHN100 | Microindentation hardness, Vickers Hardness Number at 100 g load |
| wt.% | Percentage on a weight basis |
| XRD | X-ray diffraction |
| x, y, z | Crystallographic axes |
| Z | Number of formula units per unit cell |
| 2V | Optic axial angle |
| a, b, g | Least, intermediate, and greatest refractive indices; the vibration direction of the rays; also the rays themselves. |
| a, b, g | Angles between the positive directions of the y and z, x and z, and x and y crystal axes |
| d | Birefringence |
| e | Extraordinary ray, refractive index (uniaxial) |
| o | Ordinary ray, refractive index (uniaxial) |
| □ | Vacant site in crystal structure |
Buffers
| Buffer | Description |
|---|---|
| FMQ | Fayalite + oxygen > magnetite + quartz |
| HM | Hematite—magnetite |
| IW | Iron + oxygen > wüstite |
| MH | Magnetite + oxygen > hematite |
| MI | Magnetite > iron + oxygen |
| MW | Magnetite > wüstite + oxygen |
| NNO | Nickel + oxygen > nickel oxide |
| QFM | Quartz–fayalite–magnetite |
| WM | Wüstite–magnetite |
Units of Pressure
Various units for pressure are found in geological literature. In older publications, bar or atm. (atmosphere), kilobar (kbar), and megabar (Mbar), and more recently, the SI units: pascal (Pa), megapascal (MPa), and gigapascal (GPa). Their relationships are as follows:
- 1 bar = 105 N(newtons)/m2
- 1 atm. = 1.0133 bar
- 1 kbar = 1000 bar
- 1 Pa = 1 N/m2
- 1 MPa = 106 Pa = 10 bar
- 1 GPa = 109 Pa = 10 kbar
Thus, to convert pressure in kbar to pressure in GPa, or pressure in bars to pressure in MPa, divide by 10.
FAQs
What is alumino silicate?
An aluminosilicate mineral is a type of mineral that contains both aluminum and silicon in its chemical composition.
What are examples of aluminosilicate minerals?
Examples of aluminosilicate minerals include sillimanite (Al₂SiO₅), andalusite (Al₂SiO₅), and mullite, which share similar structural features and occur in high-temperature metamorphic environments.
Is kaolinite an aluminosilicate?
Kaolinite is an aluminosilicate mineral, but it is not structurally similar to sillimanite, andalusite, or mullite. It is a clay mineral formed by weathering processes.
What is another name for aluminum silicate?
Aluminum silicate is also referred to as aluminosilicate.
What is Al2O3 SiO2 2H2O called?
This formula corresponds to kaolinite, which is a common clay mineral in the aluminosilicate group.
What is the charge of alumino silicate?
The charge of an aluminosilicate depends on its structure and composition. In sillimanite (Al₂SiO₅), the structure consists of chains of edge-sharing aluminum-oxygen octahedra, which influence the overall charge distribution.
Which group of alumino silicate minerals is widely used in making electrical insulation?
Mullite, an aluminosilicate mineral, is widely used in electrical insulation due to its high-temperature stability and excellent insulating properties.
What is the application of aluminum silicate?
Aluminum silicate minerals, such as sillimanite and mullite, are used in refractory materials, ceramics, and high-temperature applications due to their thermal stability.
What is the silicate group?
The silicate group consists of minerals that contain silicon-oxygen tetrahedra as their fundamental structural unit. In aluminosilicates, aluminum substitutes for some silicon atoms in these tetrahedra.
Is aluminium silicate used in rubber?
Yes, aluminum silicate is used in rubber to improve its physical properties, such as durability and resistance to heat.
What is the formula for China clay?
China clay, also known as kaolinite, has the chemical formula Al₂Si₂O₅(OH)₄.
Is aluminum silicate a salt?
No, aluminum silicate is not a salt. It is a mineral compound formed by the combination of aluminum, silicon, and oxygen in a crystalline structure.
What is SiO2 also known as?
SiO₂ is also known as silica, which is a common mineral found in quartz and other silicate minerals.
What is the common name for aluminum silicate?
Aluminum silicate is commonly referred to as aluminosilicate.
What is the most common silicate minerals?
The most common silicate minerals include quartz (SiO₂), feldspars, and aluminosilicates such as sillimanite, andalusite, and kyanite.
Is zeolite Aluminium silicate?
Yes, zeolites are hydrated aluminosilicates that contain aluminum and silicon in a three-dimensional framework.
What is the hardness of alumino silicate?
The hardness of aluminosilicate minerals varies; for example, sillimanite has a hardness of approximately 6.5–7 on the Mohs scale.
What are the minerals in alumino silicate clay?
Aluminosilicate clays include kaolinite, montmorillonite, and illite, which are formed through the weathering of feldspars and other silicate minerals.
What is the formula for calcium alumino silicate?
Calcium aluminosilicate has the general chemical formula CaAl₂Si₂O₈, which is found in minerals like anorthite (a feldspar).
What is the melting point of alumino silicate?
The melting point of aluminosilicates varies; for instance, mullite, a common high-temperature phase, melts at approximately 1810°C.
Is aluminium silicate a kaolin?
Kaolin is a type of aluminum silicate, specifically kaolinite, which has the chemical formula Al₂Si₂O₅(OH)₄.
Is muscovite a aluminosilicate?
Yes, muscovite is an aluminosilicate mineral that belongs to the mica group and contains potassium, aluminum, and silicon in its composition.
What is the hardest silicate?
Among silicate minerals, garnet and topaz are considered some of the hardest. In the aluminosilicate group, sillimanite has a hardness of 6.5–7 on the Mohs scale.
What are silicates give an example?
Silicates are minerals that contain silicon and oxygen as their primary components. An example is quartz (SiO₂) and aluminosilicate minerals like sillimanite (Al₂SiO₅).
What are the products of aluminum silicate?
Aluminum silicate minerals decompose or alter into products such as mullite, quartz, muscovite, sericite, pyrophyllite, kaolinite, and montmorillonite under different temperature and pressure conditions.
Is clay aluminium silicate?
Yes, clays like kaolinite and montmorillonite are composed of aluminum silicate.
What is the benefit of aluminum silicate?
Aluminum silicate minerals are beneficial for their high-temperature stability, use in ceramics, refractory materials, and as fillers in industrial applications like rubber and paint.
Why magnesium aluminum silicate?
Magnesium aluminum silicate is used in pharmaceuticals, cosmetics, and industrial applications as a thickener, binder, and stabilizer due to its unique rheological properties.
What are four uses of silicate?
Used in glass manufacturing (e.g., silica glass)
Used in ceramics and refractories (e.g., aluminosilicates)
Used in cement and construction materials
Used as fillers in paints, plastics, and rubber
Is aluminum silicate soluble in water?
No, aluminum silicate minerals are generally insoluble in water.
What is the use of aluminium silicate in paints?
Aluminum silicate is used in paints as a filler and extender to improve texture, durability, and resistance to wear.