Chapter Overview
- Water has many unique thermal and dissolving properties.
- Seawater is mostly water molecules but has dissolved substances.
- Ocean water salinity, temperature, and density vary with depth.
Water on Earth
- Presence of water on Earth makes life possible.
- Organisms are mostly water.
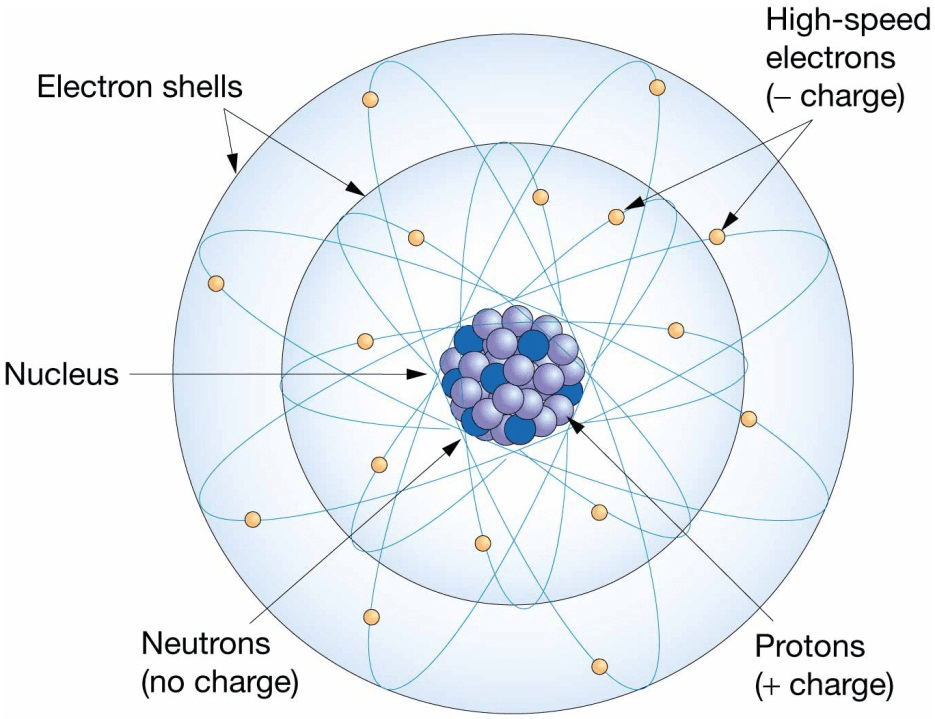
Atomic Structure
- Atoms – building blocks of all matter
- Subatomic particles
– Protons
– Neutrons
– Electrons - Number of protons distinguishes chemical elements
Molecules
– Two or more atoms held together by shared electrons
– Smallest form of a substance
Water molecule
- Strong covalent bonds between one oxygen (O) and two hydrogen (H) atoms
- Both H atoms on same side of O atom
– Bent molecule shape gives water its unique properties - Dipolar
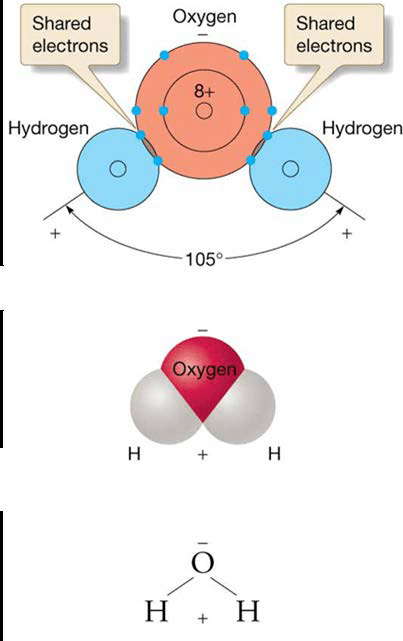
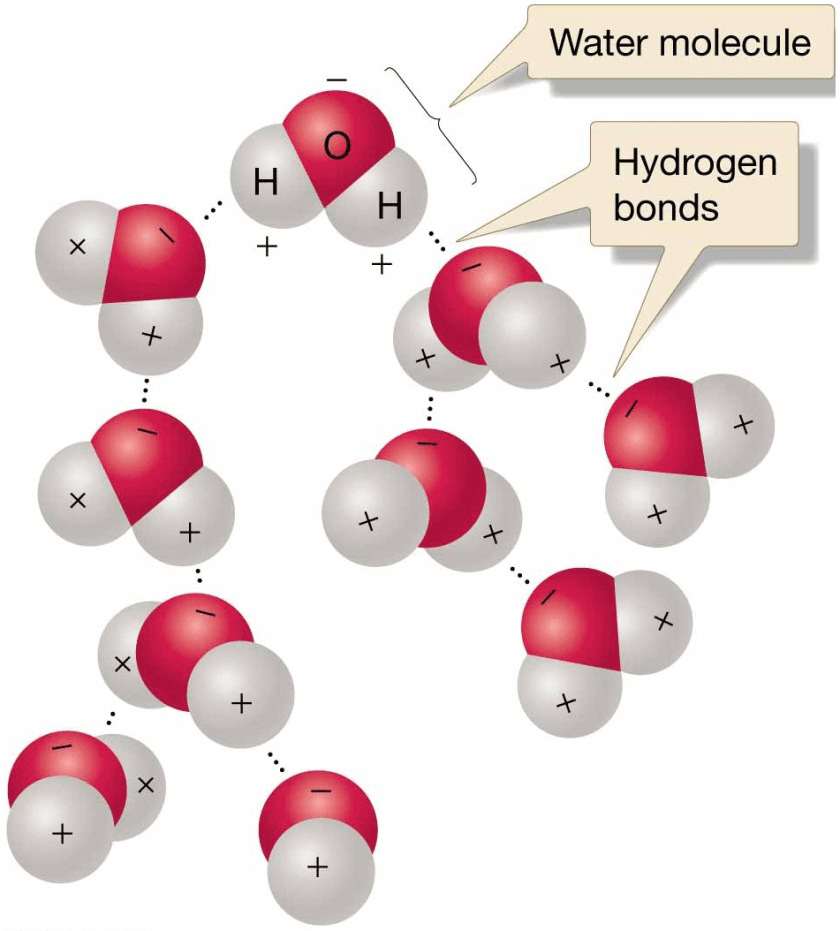
Hydrogen Bonding
- Polarity means small negative charge at O end
- Small positive charge at H end
- Attraction between positive and negative ends of water molecules to each other or other ions
- Hydrogen bonds are weaker than covalent bonds but still strong enough to contribute to
– Cohesion – molecules sticking together
– High water surface tension
– High solubility of chemical compounds in water
– Unusual thermal properties of water
– Unusual density of water
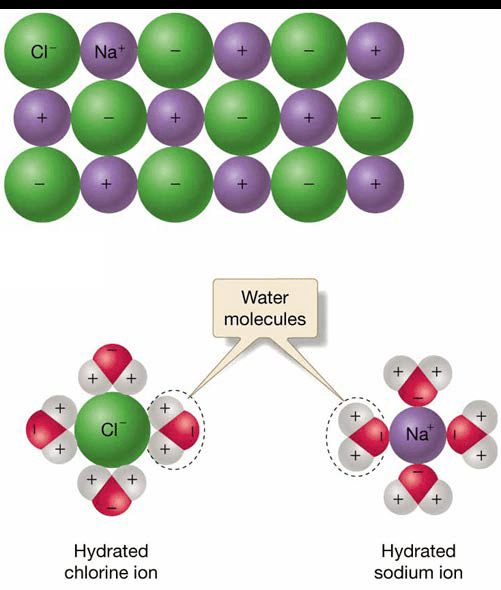
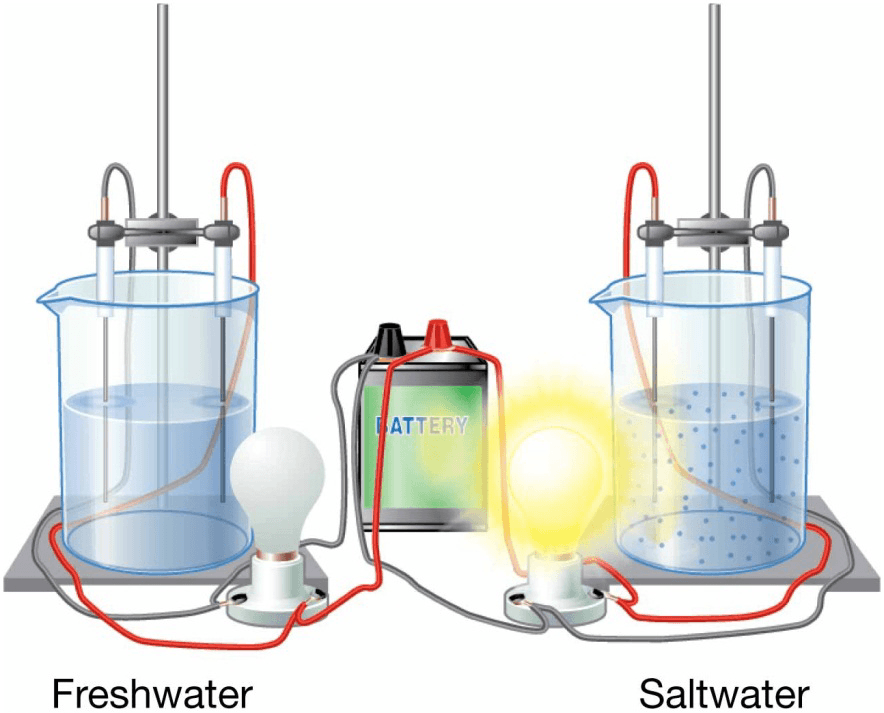
Water as Solvent
- Water molecules stick to other polar molecules.
- Electrostatic attraction produces ionic bond.
- Water can dissolve almost anything – universal solvent
Water’s Thermal Properties
Water exists on Earth as a solid, a liquid, and a gas and has the ability to store and release great amounts of heat. Water’s thermal properties influence the world’s heat budget and are in part responsible for the development of tropical cyclones, worldwide wind belts, and ocean surface currents.
- Water is solid, liquid, and gas at Earth’s surface.
- Water influences Earth’s heat budget.
Water’s Three States of Matter
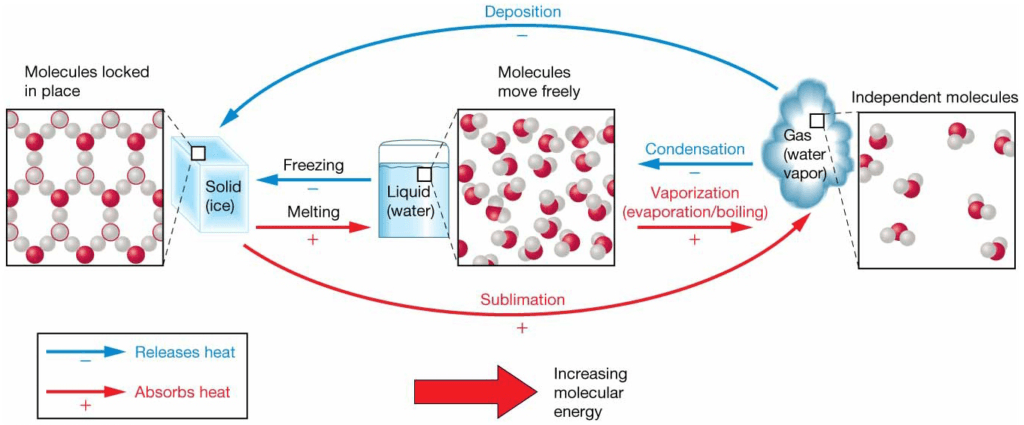
Heat, Temperature, and Changes of State
- Van der Waals forces
– Weak interactions when molecules are close together - Energy must be added for molecules to overcome attractions.
Heat and Temperature
- Heat – transfer of both kinetic and potential energy from one object to another due to temperature differences
- Temperature – average kinetic energy of molecules in a substance
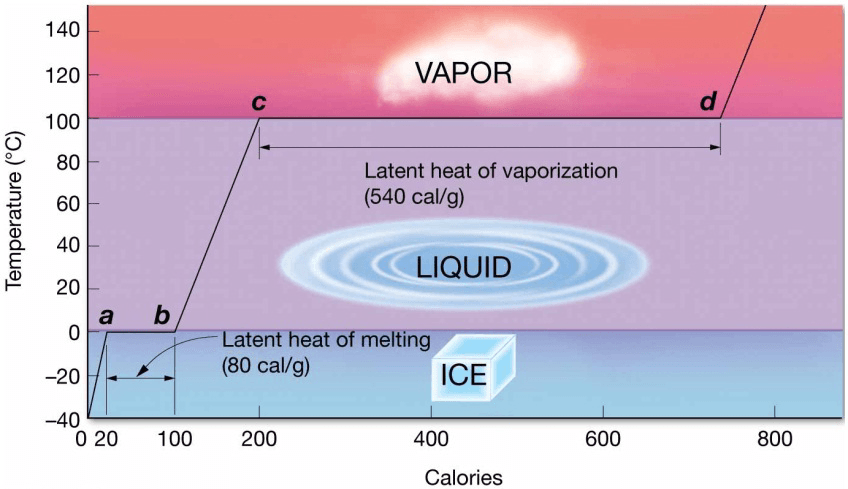
- Calorie is the amount of heat needed to raise the temperature of 1 gram of water by 1°C
Freezing and Boiling Points
- Freezing point = melting point: 0°C (32°F)
- Boiling point = condensation point: 100°C (212°F)
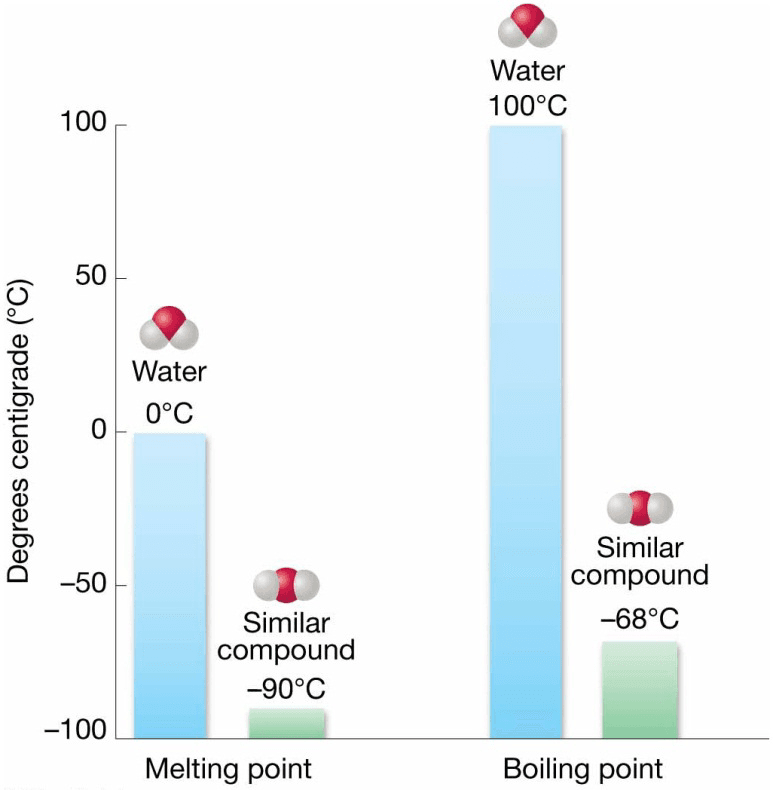
- Freezing and boiling points of water unusually high
Water’s Heat Capacity and Specific Heat
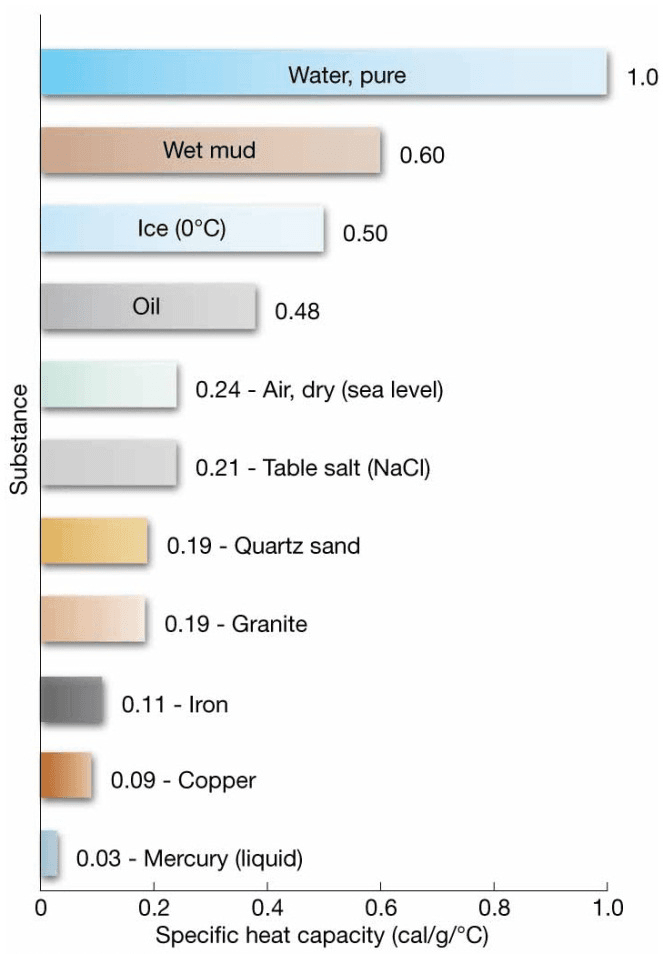
- Heat Capacity – amount of heat required to raise the temperature of 1 gram of any substance by 1°C
- Water has a high heat capacity – can take in or lose much heat without changing temperature
- Specific Heat – heat capacity per unit mass
Latent Heat
- Water has high latent heats
– Heat absorbed or released during change of state - Water’s latent heat related to its high heat capacity
- Latent Heat of Melting
– Energy needed to break intermolecular bonds that hold water molecules rigidly in place in ice crystals - Latent Heat of Vaporization
– Amount of heat that must be added to a substance at its boiling point to break the intermolecular bonds and change state from liquid to vapor
– 540 calories/gram
– All hydrogen bonds must be broken - Latent Heat of Evaporation
– Evaporation = conversion of liquid to gas below the boiling point
– 585 calories/gram
– Lower temperature of surface water not at boiling point means more hydrogen bonds to break - Latent Heat of Condensation
– Cooled water vapor turns to liquid and releases heat to the environment
– Identical to latent heat of vaporization - Latent Heat of Freezing
– Heat released when water freezes
– Identical to latent heat of melting
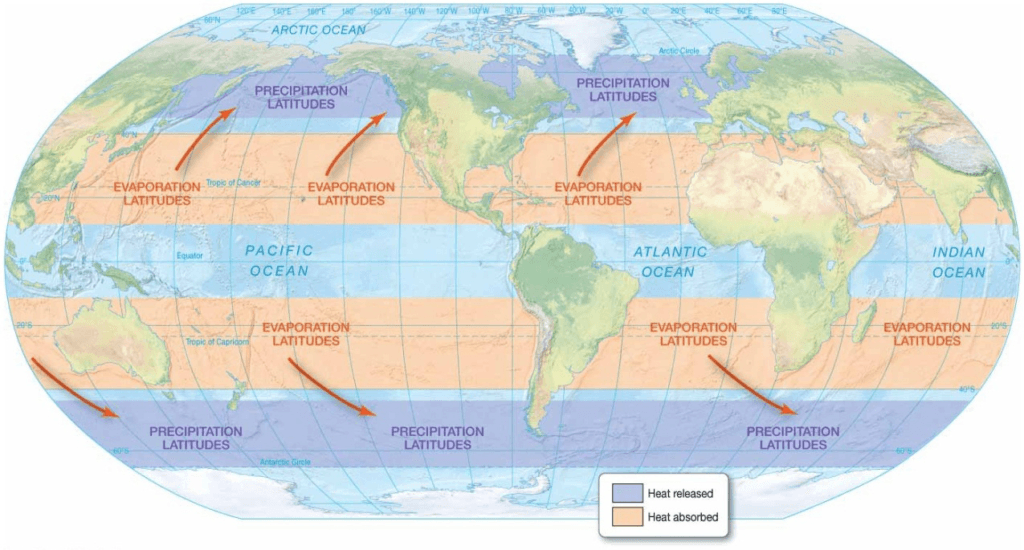
Global Thermostatic Effects
- Water’s properties moderate temperature on Earth’s surface
– Equatorial oceans do not boil
– Polar oceans do not freeze solid - Heat energy exchanged in evaporationcondensation cycle
– Makes life possible on Earth - Marine effect
– Oceans moderate temperature changes from day to night and during different seasons - Continental effect
– Land areas have greater range of temperatures from day to night and during different seasons
Day and Night Temperature Differences
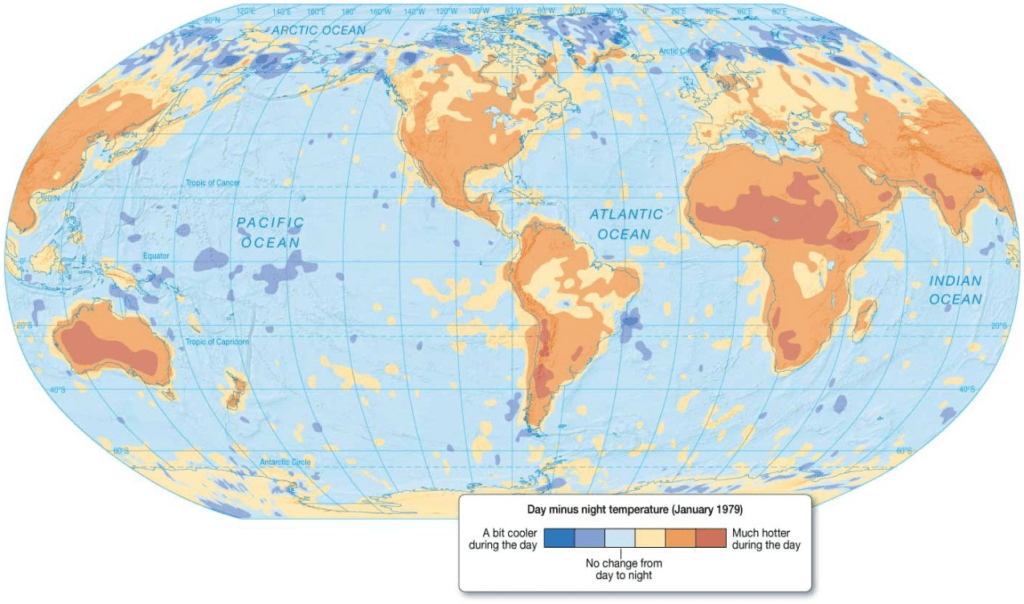
Earth. Data based on satellite measurement of the average difference in surface temperature from 2:00 p.m. to 2:00 a.m. during January 1979.
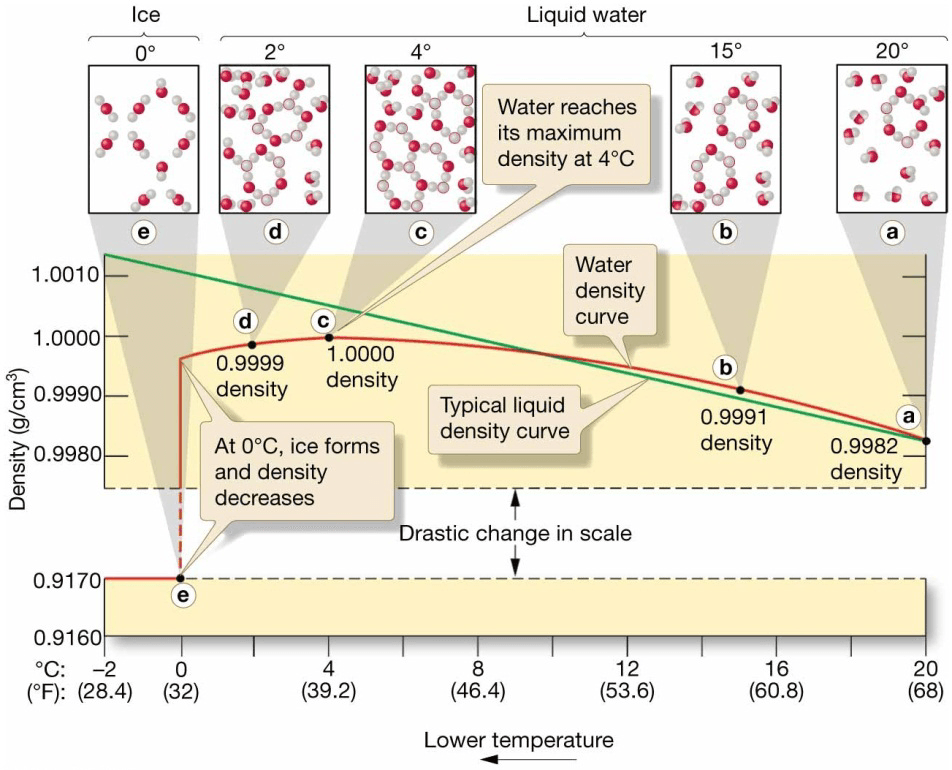
Water Density
- Density = mass/unit volume
- Density of water increases as temperature decreases.
– Thermal contraction = shrinkage of most substances caused by cold temperatures - From 4°C to 0°C the density of water decreases as temperature decreases.
– Unique property of water - Ice is less dense than liquid water.
– Changes in molecular packing
– Water expands as it freezes. - Increasing pressure or adding dissolved substances decreases the maximum density temperature.
- Dissolved solids also reduce the freezing point of water.
– Most seawater never freezes.
Salinity
Salinity (salinus = salt) is the total amount of solid material dissolved in water, including dissolved gases (because even gases become solids at low enough temperatures) but excluding dissolved organic substances. Salinity does not include fine particles being held in suspension (turbidity), or solid material in contact with water, because these materials are not dissolved. Salinity is the ratio of the mass of dissolved substances to the mass of the water sample.The salinity of seawater is typically about 3.5%, about 220 times saltier than freshwater. Seawater with a salinity of 3.5% indicates that it also contains
96.5% pure water
- Total amount of dissolved solids in water including dissolved gases
– Excludes dissolved organics - Ratio of mass of dissolved substances to mass of water sample Expressed in parts per thousand (ppt)
- Typical ocean salinity is 35 ppt (o/oo)
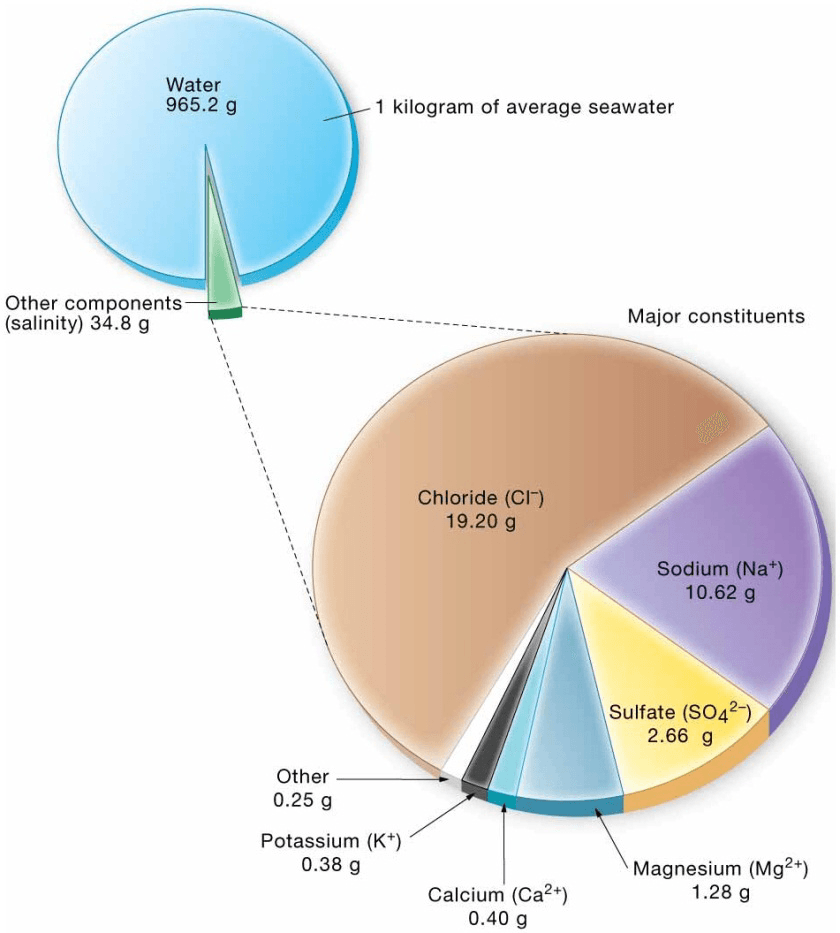
Seawater
| selected dissolved materials in 35‰ seawater | ||
| 1. Major constituents (in parts per thousand by weight, ‰) | ||
| Constituent | Concentration (‰) | Ratio of constituent/total salts (%) |
| Chloride (Cl−) | 19.2 | 55.04 |
| Sodium (Na+) | 10.6 | 30.61 |
| 2− Sulfate (SO4 ) | 2.7 | 7.68 |
| Magnesium (Mg2+) | 1.3 | 3.69 |
| Calcium (Ca2+) | 0.40 | 1.16 |
| Potassium (K+) | 0.38 | 1.10 |
| Total | 34.58‰ | 99.28% |
| 2. Minor constituents (in parts per million by weight, ppma) | |||||
| Gases | Nutrients | Others | |||
| Constituent | Concentration (ppm) | Constituent | Concentration (ppm) | Constituent | Concentration (ppm) |
| Carbon dioxide (CO2) | 90 | Silicon (Si) | 3.0 | Bromide (Br−) | 65.0 |
| Nitrogen (N2) | 14 | Nitrogen (N) | 0.5 | Carbon (C) | 28.0 |
| Oxygen (O2) | 6 | Phosphorus (P) | 0.07 | Strontium (Sr) | 8.0 |
| Iron (Fe) | 0.002 | Boron (B) | 4.6 | ||
| 3. Trace constituents (in parts per billion by weight, ppbb) | |||||
| Constituent | Concentration (ppb) | Constituent | Concentration (ppb) | Constituent | Concentration (ppb) |
| Lithium (Li) | 185 | Zinc (Zn) | 10 | Lead (Pb) | 0.03 |
| Rubidium (Rb) | 120 | Aluminum (Al) | 2 | Mercury (Hg) | 0.03 |
| Iodine (I) | 60 | Manganese (Mn) | 2 | Gold (Au) | 0.005 |
| a)Note that 1000 ppm = 1‰. b)Note that 1000 ppb = 1 ppm. | |||||
Determining Salinity
- Evaporation
– Early technique
– Weigh water and weigh evaporated salts
– Not accurate because some salts can evaporate with water - Salinometer
– Measures water’s electrical conductivity
– More dissolved substances increase conductivity - Principle of Constant Proportions
– Chemical analysis via titration
– Major dissolved constituents in same proportion regardless of total salinity
– Measure amount of halogens (Cl, Br, I, F) (chlorinity)
– Salinity = 1.80655 * Chlorinity (ppt)
Pure Water vs. Seawater
| comparison of selected properties of pure WATER AND SEAWATER | |||
| Property | Pure water | 35‰ seawater | |
| Color (light transmission) | Small quantities of water | Clear (high transparency) | Same as for pure water |
| Large quantities of water | Blue-green because water molecules scatter blue and green wavelengths best | Same as for pure water | |
| Odor | Odorless | Distinctly marine | |
| Taste | Tasteless | Distinctly salty | |
| pH | 7.0 (neutral) | Surface waters, range = 8.0–8.3; average = 8.1 (slightly alkaline) | |
| Freezing point | 0°C (32°F) | -1.9°C (28.6°F) | |
| Boiling point | 100°C (212°F) | 100.6°C (213.1°F) | |
| Density at 4°C (39°F) | 1.000 g/cm3 | 1.028 g/cm3 | |
Salinity Variations
In the open ocean far from land, salinity varies between about 33 and 38‰. In coastal areas, salinity variations can be extreme. In the Baltic Sea, for example, salinity averages only 10‰ because physical conditions create brackish (brak = salt, ish = somewhat) water. Brackish water is produced in areas where freshwater (from rivers and high rainfall) and seawater mix. In the Red Sea, on the other hand, salinity averages 42‰ because physical conditions produce hypersaline (hyper = excessive, salinus = salt) water. Hypersaline water is typical of seas and inland bodies of water that experience high evaporation rates and limited openocean circulation.
- Open-ocean salinity is 33–38 o/o.
- In coastal areas salinity varies more widely.
- Brackish
– Influx of fresh water from rivers or rain lowers salinity - Hypersaline
– High evaporation conditions
– Dead Sea - Salinity may vary with seasons (dry/rain).
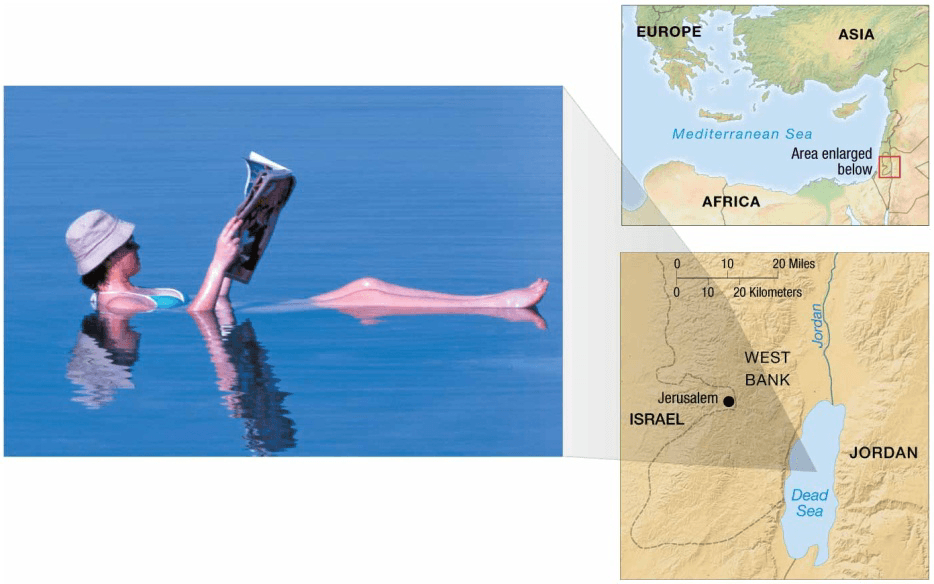
(almost 10 times the salinity of seawater), has high density. As a result, it also has high buoyancy that allows swimmers to float easily
Processes Affecting Salinity
- Decreasing salinity – adding fresh water to ocean
– Runoff, melting icebergs, melting sea ice
– Precipitation - Increasing salinity – removing water from ocean
– Sea ice formation
– Evaporation
Processes Affecting Salinity
| Processes that affect seawater salinity | ||||||
| Process | How accomplished | Adds or removes | Effect on salt in seawater | Effect on H2O in seawater | Salinity increase or decrease? | Source of freshwater from the sea? |
| Precipitation | Rain, sleet, hail, or snow falls directly on the ocean | Adds very fresh water | None | More H2O | Decrease | N/A |
| Runoff | Streams carry water to the ocean | Adds mostly fresh water | Negligible addition of salt | More H2O | Decrease | N/A |
| Icebergs melting | Glacial ice calves into the ocean and melts | Adds very fresh water | None | More H2O | Decrease | Yes, icebergs from the Antarctic have been towed to South America |
| Sea ice melting | Sea ice melts in the ocean | Adds mostly fresh water and some salt | Adds a small amount of salt | More H2O | Decrease | Yes, sea ice can be melted and is better than drinking seawater |
| Sea ice forming | Seawater freezes in cold ocean areas | Removes mostly freshwater | 30% of salts in seawater are retained in ice | Less H2O | Increase | Yes, through multiple freezings, called freeze separation |
| Evaporation | Seawater evaporates in hot climates | Removes very pure water | None (essentially all salts are left behind) | Less H2O | Increase | Yes, through evaporation of seawater and condensation of water vapor, called distillation |
Major dissolved components in streams with those in seawater
| COMPARISON OF MAJOR DISSOLVED COMPONENTS IN STREAMS WITH THOSE IN SEAWATER | ||
| Constituent | Concentration in streams (parts per million by weight) | Concentration in seawater (parts per million by weight) |
| Bicarbonate ion (HCO3 ) | 58.4 | trace |
| Calcium ion (Ca2+) | 15.0 | 400 |
| Silicate (SiO2) | 13.1 | 3 |
| Sulfate ion (SO4 ) | 11.2 | 2700 |
| Chloride ion (Cl−) | 7.8 | 19,200 |
| Sodium ion (Na+) | 6.3 | 10,600 |
| Magnesium ion (Mg2+) | 4.1 | 1300 |
| Potassium ion (K+) | 2.3 | 380 |
| Total (parts per million) | 119.2 ppm | 34,793 ppm |
| Total (‰) | 0.1192‰ | 34.8‰ |
Earth’s Hydrologic Cycle
The hydrologic cycle, (hydro = water, logos = study of) which describes the continual movement of water on, above, and below the surface of Earth. The movement of water through various components of the hydrologic cycle involves processes that recycle water among the ocean, the atmosphere, and the continents, illustrating that water is in constant motion between the
different components (or reservoirs) of the hydrologic cycle.
- Processes that affect seawater salinity
- Recycles water among ocean, atmosphere, and continents
- Water in continual motion between water reservoirs
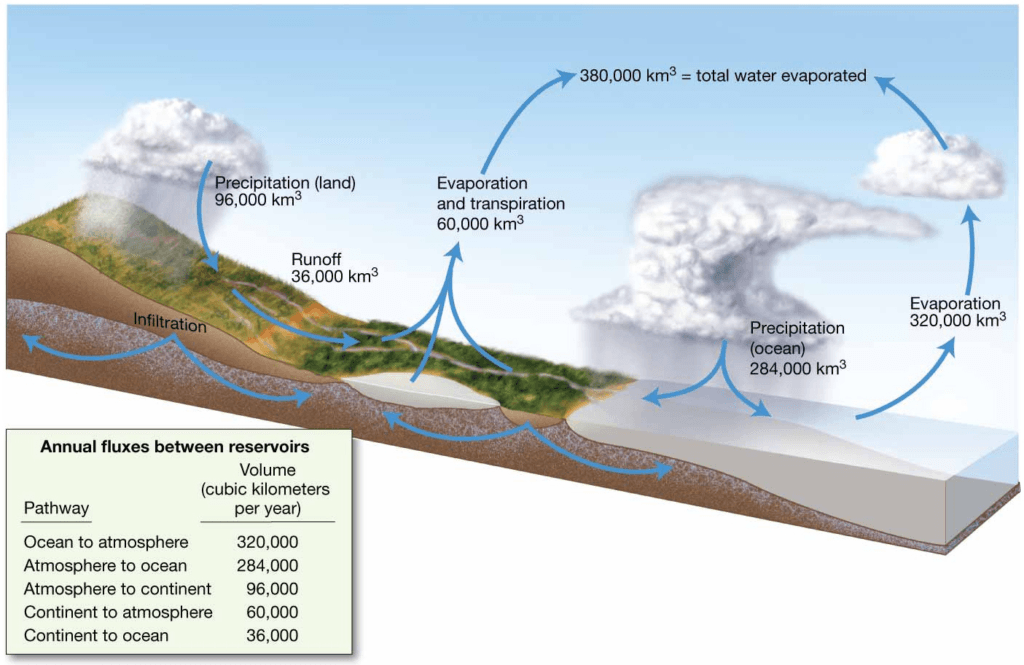
(volume of water moved between reservoirs) in cubic kilometers. Left table shows average yearly flux between reservoirs
Earth’s Water
- 97.2% in the world ocean
- 2.15% frozen in glaciers and ice caps
- 0.62% in groundwater and soil moisture
- 0.02% in streams and lakes
- 0.001% as water vapor in the atmosphere
Residence Time
- Average length of time a substance remains dissolved in seawater
- Ions with long residence time are in high concentration in seawater.
- Ions with short residence time are in low concentration in seawater.
- Steady state condition – average amounts of various elements remains constant
Processes that Add/Subtract Dissolved Substances
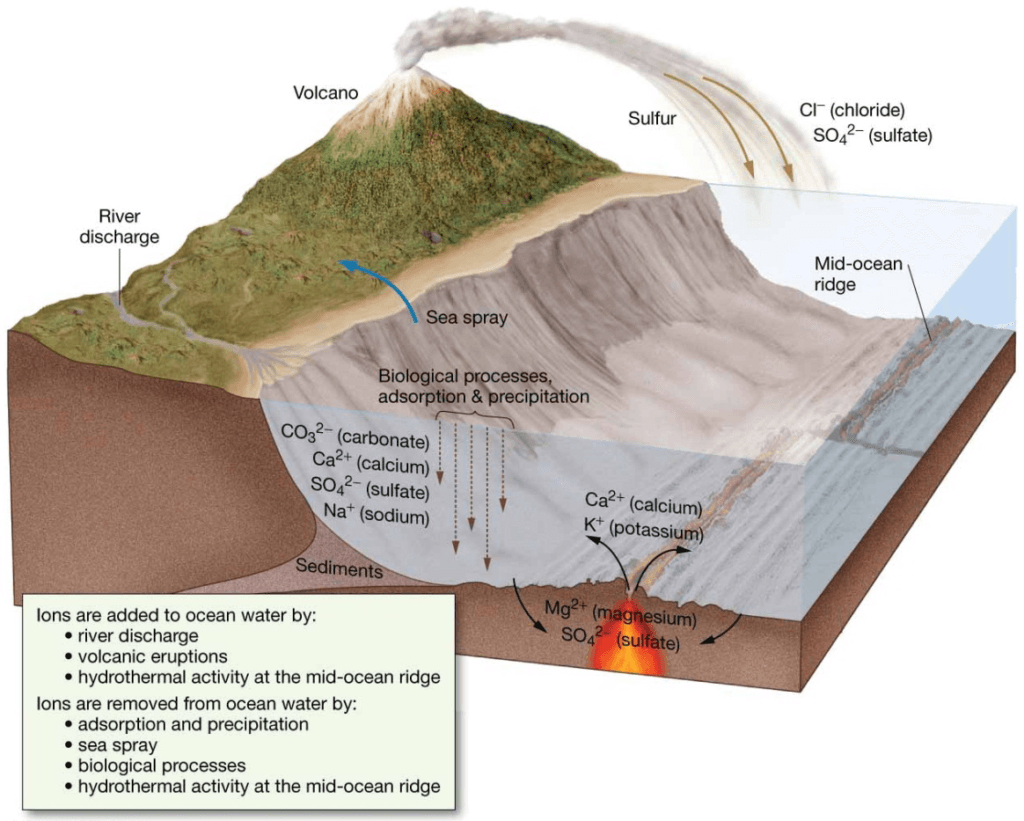
Acidity and Alkalinity
An acid is a compound that releases hydrogen ions (H⁺) when dissolved in water. The resulting solution is said to be acidic. A strong acid readily and completely releases hydrogen ions when dissolved in water.
An alkaline, or a base, is a compound that releases hydroxide ions (OH⁻) when dissolved in water. The resulting solution is said to be alkaline, or basic. A strong base readily and completely releases hydroxide ions when dissolved in water.
- Acid releases a hydrogen ion (H+) when dissolved in water.
- Alkaline (or base) releases a hydroxide ion (OH-) in water
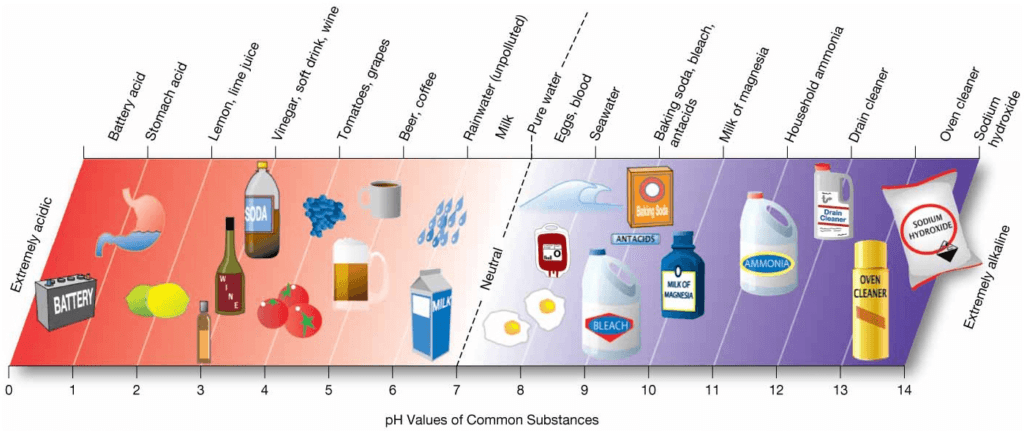
pH Scale
The pH (power of hydrogen) scale, which is a measure of the hydrogen ion concentration of a solution. Values for pH range from 0 (strongly acidic) to 14 (strongly alkaline or basic), and the pH of a neutral solution such as pure water is 7.0.
The pH scale is not linear: A decrease of 1.0 pH unit corresponds to a 10-fold increase in the concentration of hydrogen ions, making the water more acidic, whereas a change of 1.0 unit upward corresponds to a 10-fold decrease, making the water more alkaline.
Ocean surface waters have a pH that averages about 8.1 and ranges from about 8.0 to 8.3, so seawater is slightly alkaline.
- Measures hydrogen ion concentration
– pH value less than 7 = acid
– pH value greater than 7 = base (alkaline)
– pH 7 = neutral - Pure water
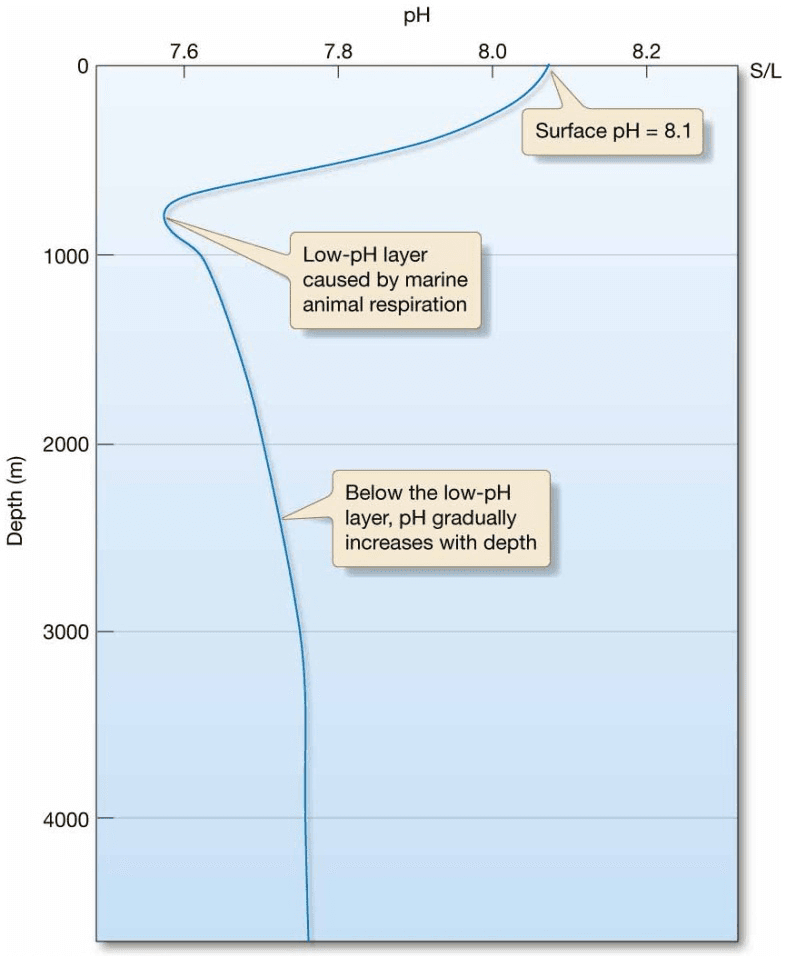
Ocean pH
- Seawater is slightly alkaline
– Surface water average pH 8.1 - Ocean water pH decreases with depth
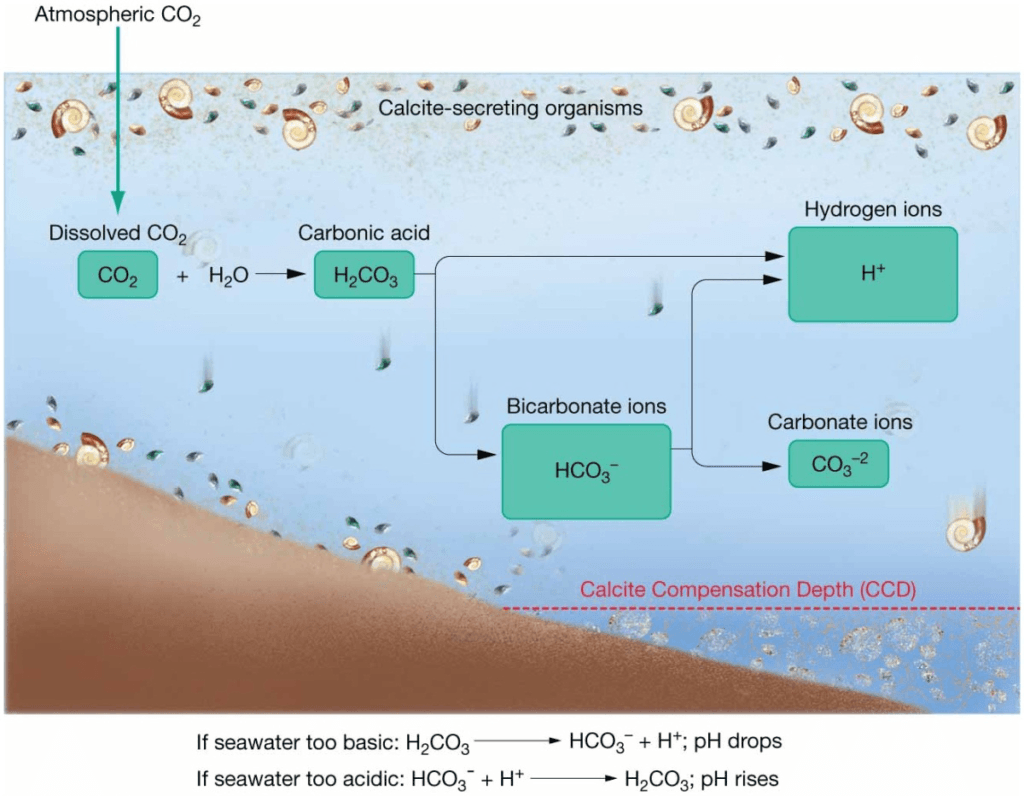
Carbonate Buffering System
- Buffering keeps the ocean from becoming too acidic or too basic.
- Precipitation or dissolution of calcium carbonate, CaCO3, buffers ocean pH.
- Oceans can absorb CO2 from the atmosphere without much change in pH.
Surface Salinity Variation
- High latitudes
– Low salinity
– Abundant sea ice melting, precipitation, and runoff - Low latitudes near equator
– Low salinity
– High precipitation and runoff - Mid latitudes
– High salinity
– Warm, dry, descending air increases evaporation
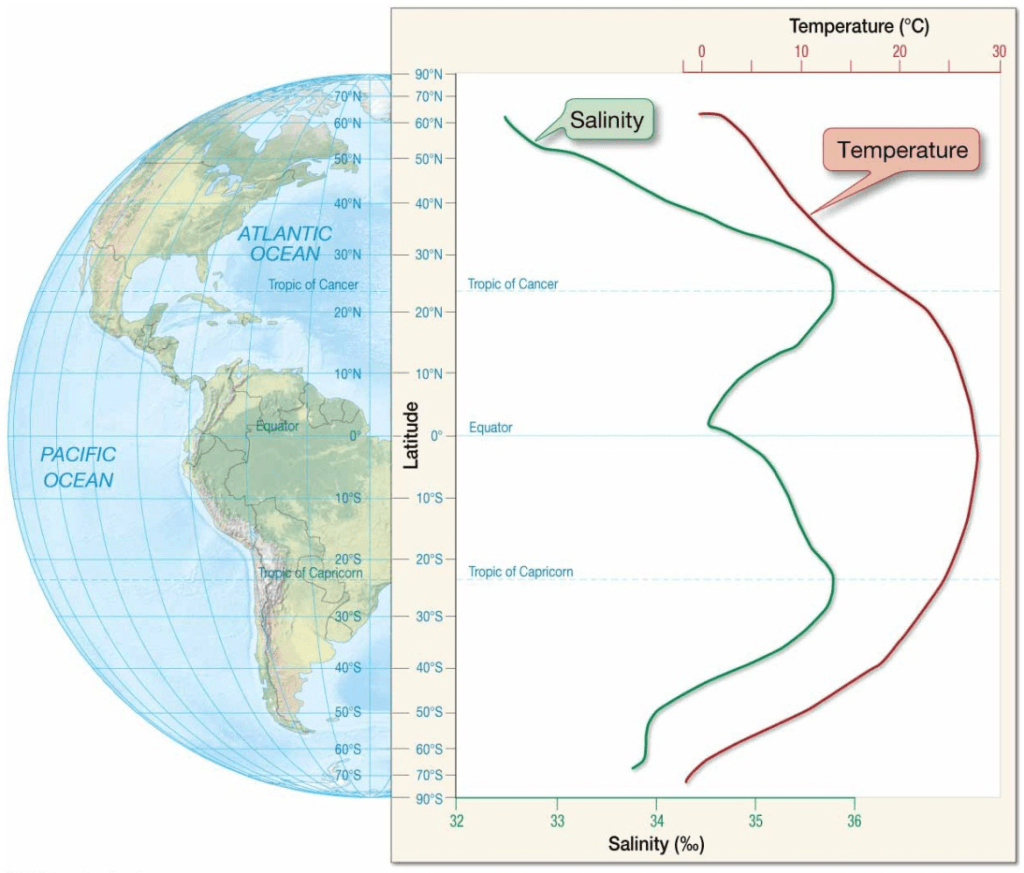
surface salinity (green curve) along with sea surface temperature (red curve).
Salinity Variation with Depth
- Low latitudes – salinity decreases with depth
- High latitudes – salinity increases with depth
- Deep ocean salinity fairly consistent globally
- Halocline – separates ocean layers of different salinity
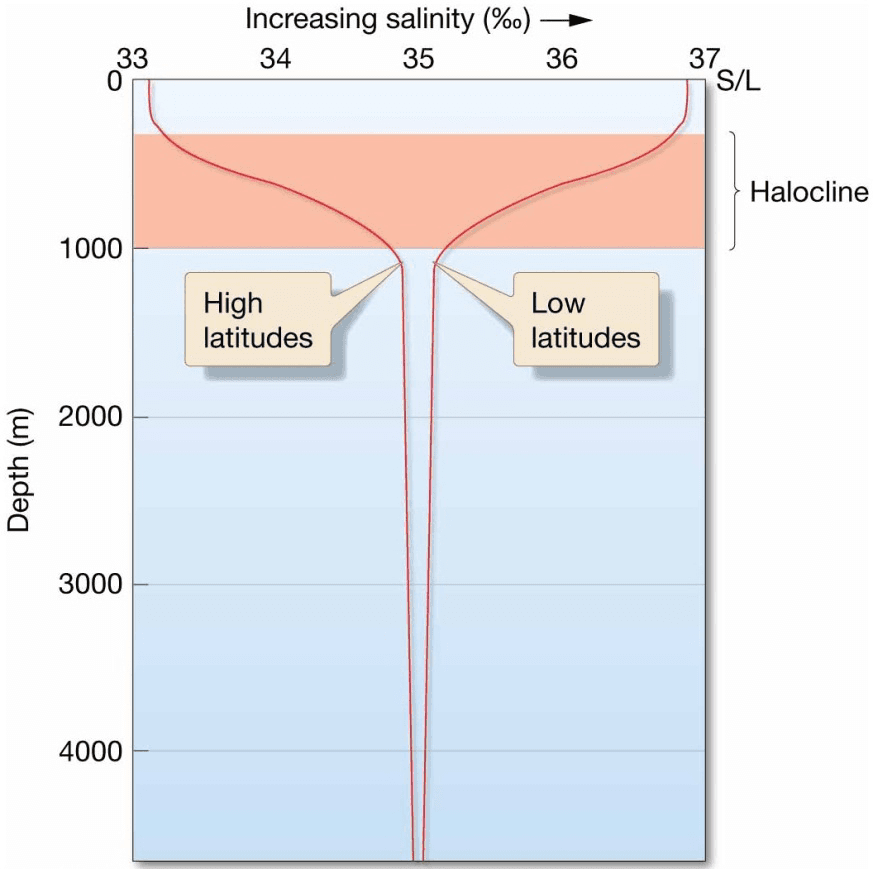
Graph showing a vertical profile of high- and low-latitude salinity variation with depth. Horizontal scale is in ‰; vertical scale is depth in meters, with sea level at the top. The layer of rapidly changing salinity is called the halocline.
Seawater Density
- Freshwater density = 1.000 g/cm3
- Ocean surface water =1.022 to 1.030 g/cm3
- Ocean layered according to density
- Density increases with decreasing temperature
– Greatest influence on density - Density increases with increasing salinity
- Density increases with increasing pressure
– Does not affect surface waters
Factors Affecting Seawater Density
The ocean, like Earth’s interior, is layered according to density. Low-density water exists near the surface, and higher-density water occurs below. Except for some shallow inland seas with high rates of evaporation that create high-salinity water, the highest-density water is found at the deepest ocean depths. Let’s examine how temperature, salinity, and pressure influence seawater density by expressing the relationships using arrows (up arrow = increase; down arrow = decrease):
- As temperature increases (↑), seawater density decreases (↓) due to thermal expansion.
- As salinity increases (↑), seawater density increases (↑) due to the addition of more dissolved material.
- As pressure increases (↑), seawater density increases (↑) due to the compressive effects of pressure.
Of these three factors, only temperature and salinity influence the density of surface water. Pressure influences seawater density only when very high pressures are encountered, such as in deep-ocean trenches. Still, the density of seawater in the deep ocean is only about 5% greater than at the ocean surface, showing that despite tons of pressure per square centimeter, water is nearly incompressible.
Temperature and Density Variations With
Depth
- Pycnocline – abrupt change of density with depth
- Thermocline – abrupt change of temperature with depth
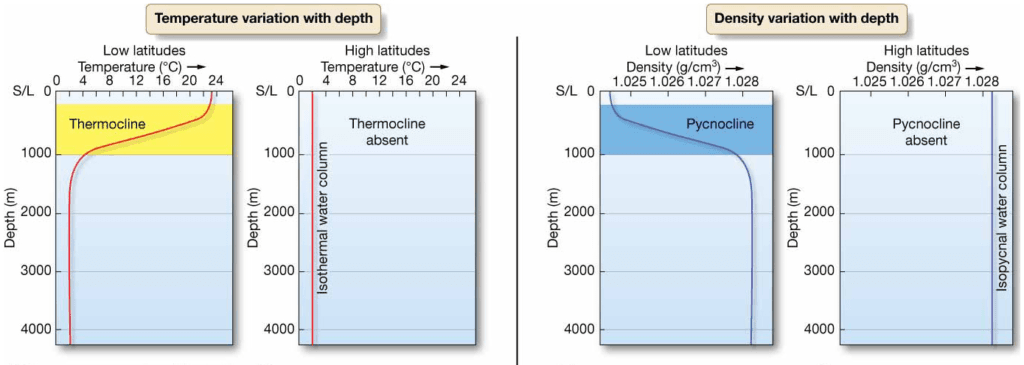
Layered Ocean
Three distinct water masses based on density:
- Mixed surface layer – above thermocline
- Upper water – thermocline and pycnocline
- Deep water – below thermocline to ocean floor
- High latitude oceans – thermocline and pycnocline rarely develop
– Isothermal – no temperature variation in water column
– Isopycnal – no density variation in water column
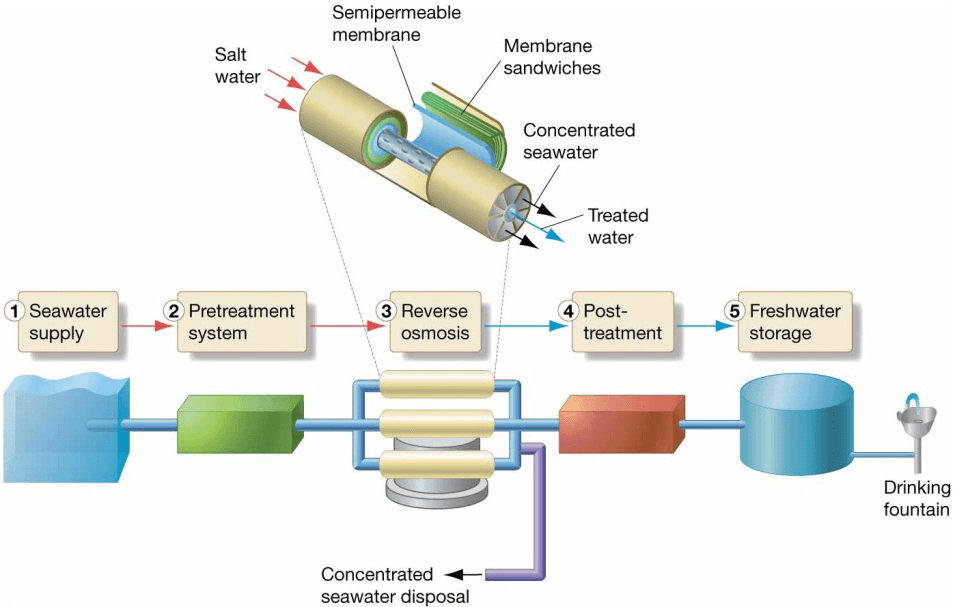
Desalinization
- Removing salt from seawater
- Human need for fresh water increasing, water
supply decreasing - Energy-intensive and expensive
- Most desalinization plants in arid regions
– Provide less than 0.5% of human water needs - Distillation
– Most common process
– Water boiled and condensed
– Solar distillation in arid climates
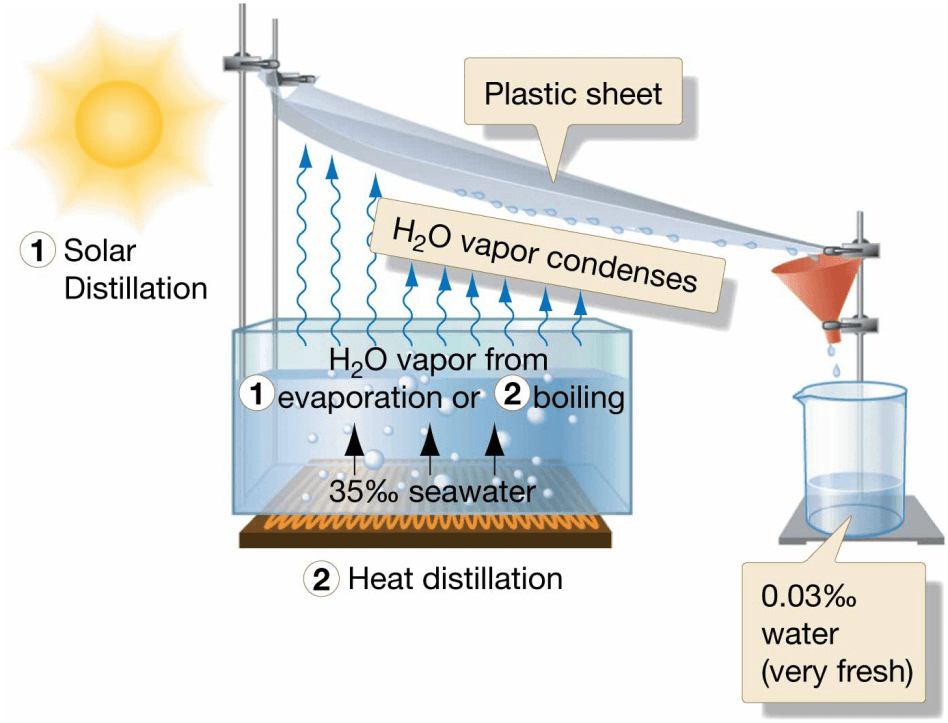
- Electrolysis
– Electrode-containing freshwater
– Membrane between fresh and salt water tanks
- Reverse osmosis
– Salt water forced through membrane into fresh water - Freeze separation
– Water frozen and thawed multiple times
References
All content and images in this article are sourced from the following:
Turcillo, A. R. Essentials of Oceanography.
Reference: All images and content are taken from Essentials of Oceanography by Alan P. Trujillo and Harold V. Thurman, 12th Edition.