Biogenous sediment (from bio, meaning life, and generare, meaning to produce)—also known as biogenic sediment—originates from the preserved hard remains of once-living organisms.
Origin of Biogenous Sediment
Biogenous sediment initially forms from the durable components (such as shells, bones, and teeth) of various living organisms, which range in size and complexity from microscopic algae and protozoa to marine vertebrates like fish and whales. Upon the death of these organisms, their remnants descend to the seafloor, progressively accumulating to generate biogenous sediment.
This type of sediment can be grouped into two main categories: macroscopic and microscopic. Macroscopic biogenous sediment includes large fragments that are visible to the naked eye, such as shells, bones, and teeth of larger organisms. Although prominent in certain tropical coastal areas—notably where shells and coral fragments are abundant—this form of sediment is generally uncommon in most marine environments, especially in deep-sea regions, where biological populations tend to be lower.
Far more prevalent is the microscopic biogenous sediment, consisting of extremely fine particles that require microscopic observation for detailed visibility. These originate from minute organisms that form tiny shells, termed tests (from testa, meaning shell). Following the organism’s death, these delicate tests gradually descend through the water column, steadily accumulating in large quantities on the ocean bed.
Over time, these microscopic tests contribute to the formation of distinctive sediment deposits known as ooze (derived from wose, meaning juice). As the name suggests, ooze possesses a fine-grained, mushy texture. To be classified as biogenous ooze, a deposit must consist of at least 30% biogenous test material by weight. The remaining portion, which may comprise up to 70%, typically consists of fine lithogenous clay intermixed during deposition in deep-marine settings. By volume, microscopic ooze covers a significantly greater portion of the ocean floor than macroscopic biogenous sediment, making it a fundamental component of deep-ocean sedimentary processes.
Organisms and Composition of Biogenous Sediment
The primary contributors to biogenous sediment are specific categories of marine life—most notably algae (alga = seaweed) and protozoans (proto = first, zoa = animal). Algae represent a group of predominantly aquatic, eukaryotic, and photosynthetic organisms, whose size ranges from microscopic unicellular forms to macroscopic species such as giant kelp. In contrast, protozoans encompass a diverse array of single-celled, eukaryotic, and generally non-photosynthetic organisms, most of which are microscopic in scale.
Composition of Biogenous Sediment
The two most fundamental chemical constituents of biogenous sediment are calcium carbonate (CaCO₃, forming the mineral calcite) and silica (SiO₂). Silica, in many instances, occurs in its hydrated form—SiO₂·nH₂O—commonly referred to as opal.
Silica-Based Components
A significant portion of the silica found in biogenous ooze originates from microscopic algae known as diatoms (diatoma = cut in half) and protozoans called radiolarians (radio = a spoke or ray). Due to their photosynthetic nature, diatoms require access to intense sunlight and therefore inhabit only the sunlit upper layers of the ocean. Most diatoms exist in a planktonic state (planktos = wandering), meaning they drift freely within the photic zone.
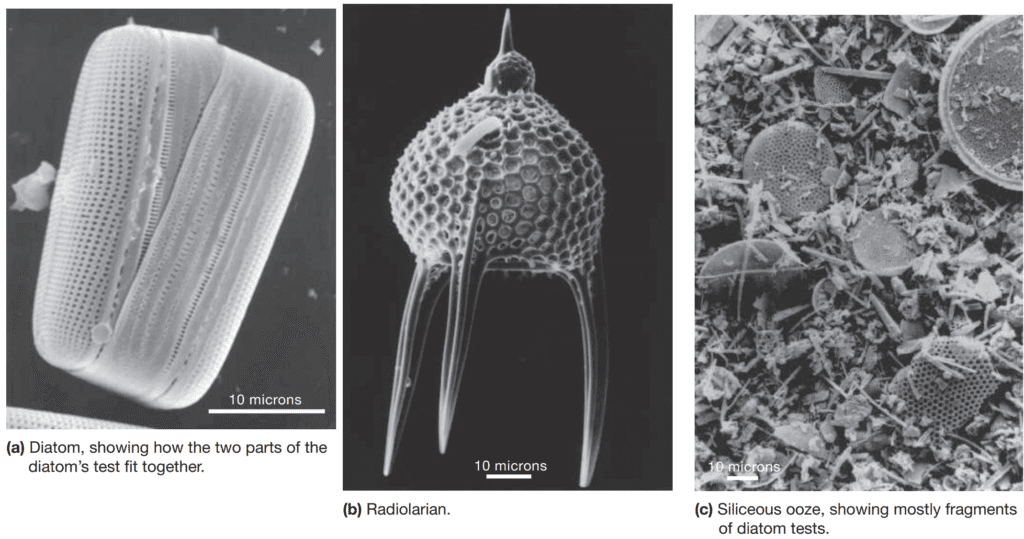
These organisms construct intricate silica-based structures resembling glass greenhouses, which serve as protective casings. The typical diatom test consists of two interlocking parts, comparable to a petri dish or pillbox, that enclose the living cell. These siliceous tests are decorated with elaborate perforations to facilitate the exchange of nutrients and expulsion of metabolic waste.
In regions where diatom populations flourish at the ocean surface, substantial accumulations of diatom-rich ooze can develop on the seafloor below. Upon lithification, this ooze transforms into diatomaceous earth—a lightweight, white sedimentary rock composed primarily of diatom tests mixed with clay.
Radiolarians are microscopic, unicellular protozoans, the majority of which exhibit a planktonic lifestyle. As suggested by their etymology, these organisms often possess elongated spines or ray-like extensions made of silica, projecting from their siliceous exoskeleton. Although radiolarians are incapable of photosynthesis, they depend on external nutritional sources, primarily consuming bacteria and other planktonic organisms.
A notable feature of radiolarians is their consistently well-developed radial symmetry, which has earned them the poetic epithet, the “living snowflakes of the sea.” The persistent deposition of siliceous tests—originating from diatoms, radiolarians, and other silica-secreting marine organisms—results in the formation of siliceous ooze.
Calcium Carbonate Components
Two primary contributors to calcium carbonate-rich biogenous ooze are foraminifers (foramen = an opening) and coccolithophores (coccus = berry, lithos = stone, phorid = carrying). Foraminifers are closely related to radiolarians and similarly consist of microscopic, planktonic protozoans, while coccolithophores are photosynthetic, single-celled algae that generate calcium carbonate plates. These calcareous structures contribute substantially to biogenous marine sediment, particularly in nutrient-rich surface waters and shallow marine environments, where photosynthetic activity is most intense.
Coccolithophores are unicellular algae, the majority of which exist in a planktonic state. These photosynthetic organisms generate thin calcareous plates known as coccoliths, composed of calcium carbonate. Typically, between 20 to 30 coccoliths interlock and overlap to form a spherical outer shell, or test, that encases the organism.
As with diatoms, coccolithophores rely on sunlight for photosynthesis, and thus inhabit the sunlit surface layers of the ocean. However, coccolithophores are extremely small, typically ranging from 10 to 100 times smaller than the average diatom. Owing to their minute size, they are often referred to as nannoplankton (nanno = dwarf, planktos = wandering).
Upon the death of the organism, the coccolith plates separate and disperse, gradually settling on the ocean floor to form coccolith-rich ooze. Over extended periods, this ooze undergoes lithification, transforming into a soft, white sedimentary rock known as chalk. Chalk has a variety of practical applications, one of the most familiar being its historical use for writing on chalkboards.
An iconic example of such a calcium carbonate deposit is the White Cliffs of southern England, which are composed of solidified coccolith ooze that was originally deposited on the ocean bottom and subsequently uplifted onto land. Comparable chalk formations of the same geologic age are widely distributed across Europe, North America, Australia, and the Middle East. These widespread deposits are so notable that the geologic era during which they were formed has been designated the Cretaceous Period (creta = chalk).
Foraminifers are unicellular protozoans, many of which exhibit a planktonic lifestyle, and vary in scale from microscopic to macroscopic dimensions. Unlike photosynthetic organisms, foraminifers lack the ability to synthesize their own food and thus depend on the ingestion of external organisms for nutritional sustenance.
These organisms construct rigid calcium carbonate shells, referred to as tests, which serve as protective habitats for the living cell. The majority of foraminifers develop chambered or segmented tests, each featuring a distinctive aperture at one end. Though generally small in size, their calcified tests bear a close resemblance to the larger marine shells commonly observed along coastal shorelines.
Sediment accumulations consisting predominantly of foraminifer tests, coccoliths, and other calcareous-producing organisms are termed calcareous ooze. These deposits form a significant component of marine sedimentary environments, especially in open-ocean settings.
Distribution of Biogenous Sediment
Among the various pelagic sediment types, biogenous sediment is one of the most prevalent on the deep-ocean floor. Its distribution pattern is governed primarily by three critical factors: (1) productivity, (2) destruction, and (3) dilution.
Productivity refers to the concentration of biological organisms present in the surface waters directly above the seafloor. Regions characterized by high biological productivity exhibit abundant life forms, increasing the likelihood of biogenous sediment accumulation. Conversely, in low-productivity zones, the scarcity of organisms results in insufficient deposition of biogenous ooze.
Destruction involves the dissolution of skeletal remains (or tests) in seawater—a process influenced by depth and chemical conditions. In some circumstances, these remains may dissolve before they ever descend to the ocean bottom. In others, dissolution occurs after reaching the seabed, thereby preventing their consolidation into sedimentary deposits.
Carbonate Deposits
Carbonate-based minerals are defined by the presence of the CO₃ group in their molecular structure—for example, calcium carbonate (CaCO₃). Geological formations originating in marine settings that are predominantly composed of calcium carbonate are identified as limestones. A substantial proportion of these limestones incorporate fossilized remnants of marine organisms, implying a biogenous derivation. However, other rocks containing carbonate appear to have precipitated inorganically from ocean water, independent of biological processes. Present-day depositional settings—such as the Bahama Banks, the Great Barrier Reef off Australia, and the Persian Gulf—indicate that carbonate accumulation predominantly occurs in shallow, tropical marine zones, particularly on continental shelves and surrounding equatorial islands, where coral reefs and coastal beaches dominate the landscape.
Historically, marine carbonate formations make up approximately 2% of Earth’s outer layer and comprise nearly 25% of all sedimentary rock types globally. Notably, vast expanses of marine limestone serve as the foundation bedrock in regions like Florida and across numerous Midwestern U.S. states, including Kentucky, Michigan, Pennsylvania, and Colorado. The chemical weathering of these deposits via groundwater percolation has led to the dissolution of limestone, thereby giving rise to sinkholes and, in certain instances, the creation of extensive cave systems exhibiting remarkable geologic features.
Stromatolites
Stromatolites are dome-shaped formations consisting of finely laminated carbonate layers, typically developed in unique shallow marine environments with elevated salinity, such as the tidal pools of Shark Bay, Western Australia. These biosedimentary structures are generated primarily by cyanobacteria, which secrete mucilaginous films that ensnare fine-grained sediment. Additional species of algae contribute by extending filamentous networks that facilitate the cohesion of carbonate particles. Much like the progressive growth of tree rings, these microbial colonies expand upward layer by layer, constructing a bulbous, stratified framework. Geological evidence indicates that conditions between approximately 1 to 3 billion years ago were highly favorable for stromatolite proliferation, resulting in ancient formations that today reach several hundred meters in height, preserved within stratified rock sequences from those time periods.
Pelagic Deposits
On the deep-sea floor, microscopic biogenous sediment—referred to as ooze—is frequently encountered. This prevalence is attributed to the minimal presence of lithogenous sediment at such remote distances from continental sources, which otherwise might dilute the concentration of biogenous matter.
Siliceous Ooze
Siliceous ooze is characterized by a composition containing at least 30% of the durable remains of silica-secreting organisms. When this ooze is predominantly formed from diatoms, it is termed diatomaceous ooze. If primarily composed of radiolarians, it is designated radiolarian ooze. In cases where the material consists chiefly of single-celled silicoflagellates—a distinct type of protozoan—the deposit is referred to as silicoflagellate ooze.
Throughout the ocean, silica is present in undersaturated concentrations at all depths, indicating that solid silica particles are inherently unstable and prone to dissolution in seawater. Consequently, in the absence of continual production by living diatoms, radiolarians, and silicoflagellates, their siliceous tests would inevitably dissolve. The degradation of these biogenous siliceous components—that is, the skeletal remains of deceased organisms slowly descending to the ocean bottom—occurs gradually and persistently at every depth due to this dissolving process.

Yet, the formation of siliceous ooze on the seabed is still possible despite this constant dissolution. One effective mechanism involves the rapid deposition of siliceous tests in concentrations faster than seawater can chemically erode them. For example, when a substantial number of tests sink simultaneously, they can collectively form a sedimentary layer of siliceous ooze. Upon being buried beneath successive layers, these tests become shielded from direct interaction with seawater, halting their dissolution. As a result, siliceous ooze tends to accumulate in regions beneath surface waters with notably high biological productivity involving silica-secreting organisms.
Calcareous Ooze and the CCD
Calcareous ooze is defined as a sediment containing at least 30% of the solidified remains of calcium carbonate-secreting organisms. When this ooze is composed chiefly of coccolithophores, it is termed coccolith ooze. If the dominant component consists of foraminifers, the deposit is called foraminifer ooze. Among the most widespread varieties of foraminifer ooze is Globigerina ooze, named after a specific foraminifer that occurs extensively in the Atlantic and South Pacific Oceans. Other categories of calcareous ooze include pteropod oozes and ostracod oozes.
The dissolution behavior of calcium carbonate (in the form of calcite) is highly dependent on ocean depth. In warmer surface waters and in shallow marine zones, the seawater typically remains saturated with calcium carbonate, thereby inhibiting dissolution. However, in the deep ocean, where temperatures are significantly lower, the water holds a higher concentration of carbon dioxide. This increased CO₂ reacts to form carbonic acid, which actively promotes the breakdown of calcareous material. Furthermore, the elevated pressure found at greater depths contributes to the accelerated dissolution of calcium carbonate.
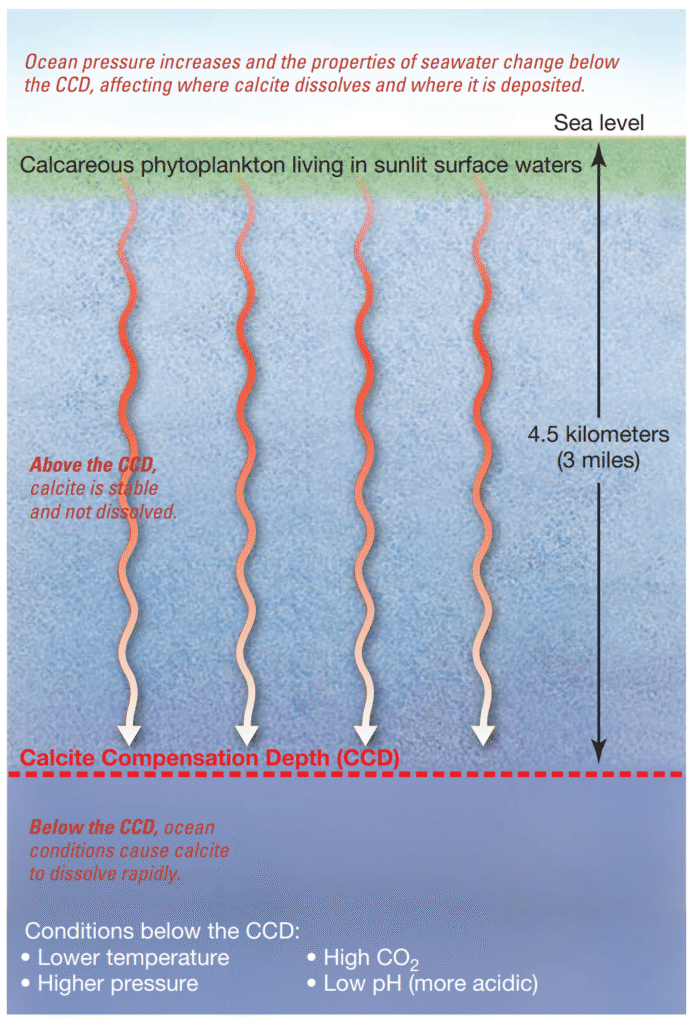
The specific depth in the ocean where hydrostatic pressure becomes sufficiently intense and carbon dioxide concentrations in deep-sea water are substantial enough to initiate the dissolution of calcium carbonate is referred to as the lysocline (lusis meaning “a loosening,” and cline meaning “slope”).
Beneath the lysocline, calcium carbonate begins to dissolve at an accelerated rate as depth increases, continuing until it reaches the calcite compensation depth (CCD). At or below the CCD, sediments typically contain minimal calcite, as it dissolves rapidly—often even the robust tests of foraminifers are entirely disintegrated within one to two days. Essentially, calcite can accumulate only on the summits of elevated submarine features that rise above the CCD, while it dissolves at depths situated near the base of these underwater peaks. This phenomenon is analogous to a mountain snow line, except in this case, it involves the deposition of pale calcite particles atop seamounts instead of ice and snow.
The average depth of the CCD is approximately 4500 meters (15,000 feet) beneath sea level. However, due to variations in deep-ocean chemical composition, it can extend as deep as 6000 meters (20,000 feet) in parts of the Atlantic Ocean or occur at shallower depths—around 3500 meters (11,500 feet)—in the Pacific Ocean. Likewise, the lysocline’s position is not constant across ocean basins but typically occurs at a depth of about 4000 meters (13,100 feet).
Throughout the geologic past, elevated levels of carbon dioxide in the atmosphere have resulted in greater amounts of dissolved CO₂ within the ocean, thereby increasing acidity and causing the calcite compensation depth (CCD) to rise upward.
At present, scientific studies have verified a continued increase in ocean acidity, attributed to heightened atmospheric carbon dioxide resulting from anthropogenic emissions. The relationship between ocean acidification and its impact on marine organisms is examined in detail in the section titled “The Oceans and Climate Change.” Due to the influence of the CCD, modern calcareous oozes are typically scarce at depths exceeding 5000 meters (16,400 feet). Nevertheless, subsurface layers of ancient calcareous ooze are frequently located beneath the CCD. This raises the question: how can calcareous sediments persist below the dissolution threshold?
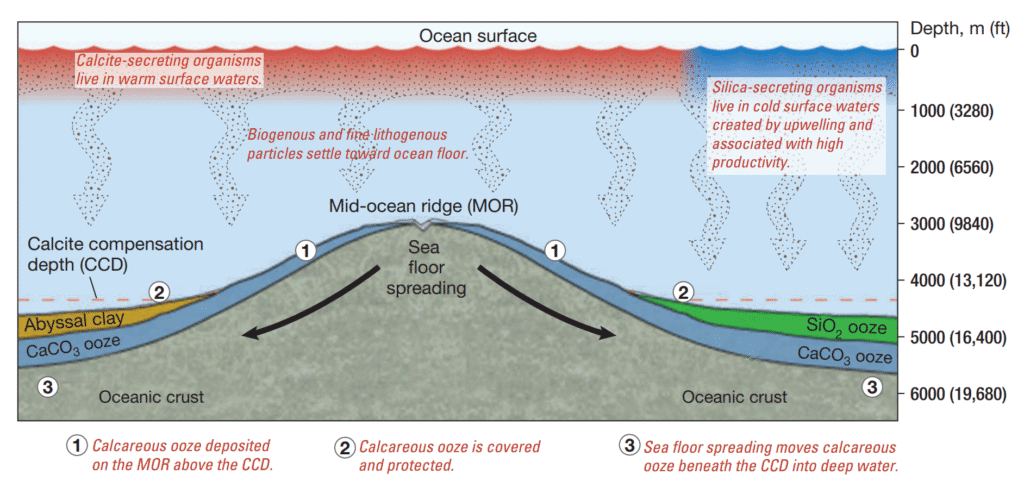
The conditions necessary to explain this are illustrated conceptually. The mid-ocean ridge is a topographic elevation on the ocean floor that extends significantly above the surrounding seabed. It frequently projects above the CCD, even when adjacent deep-sea regions lie below it. As a result, calcareous ooze that accumulates atop the mid-ocean ridge remains intact, protected from dissolution. Over time, however, seafloor spreading causes both the newly formed oceanic crust and its overlying carbonate sediments to gradually migrate away from the ridge axis, moving into deeper waters where they are eventually transported beneath the CCD.
These calcareous sediments are prone to dissolution once they descend below the CCD, unless they become buried beneath other sedimentary layers that remain unaffected by calcite dissolution, such as siliceous ooze or abyssal clay.
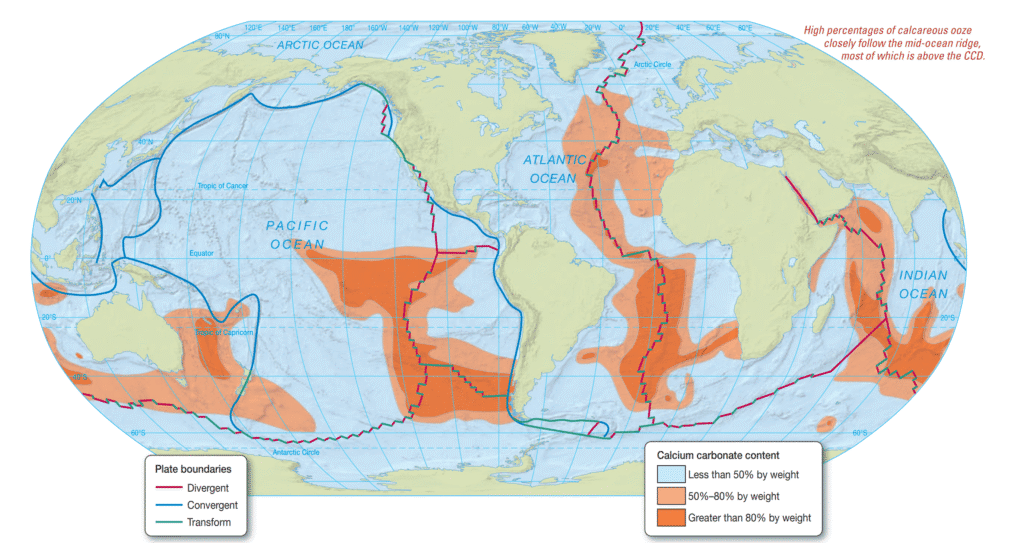
A spatial representation (referenced in the associated figure) illustrates the percentage by weight of calcium carbonate present in modern surface sediments across the major ocean basins. Elevated concentrations of calcareous ooze—occasionally surpassing 80%—are typically found along portions of the mid-ocean ridge. In contrast, such carbonate-rich sediments are scarce within the deep-ocean basins that lie beneath the CCD. For instance, the northern Pacific Ocean, recognized as one of the deepest oceanic regions globally, exhibits minimal calcium carbonate within its sediment profile. Similarly, calcareous material is uncommon in deposits forming beneath cold, high-latitude waters, where the abundance of carbonate-secreting organisms is relatively limited.
A comparative summary of environmental characteristics derived from siliceous and calcareous oozes is presented in tabular form. The data indicate that siliceous ooze predominantly develops in regions characterized by cool surface waters, especially in upwelling zones where nutrient-rich deep waters rise to the surface, enhancing biological productivity. In contrast, calcareous ooze generally accumulates on shallower oceanic terrains situated beneath warmer surface waters, reflecting the preferential habitat of calcium carbonate-producing organisms.
COMPARISON OF ENVIRONMENTS INTERPRETED FROM DEPOSITS OF SILICEOUS AND CALCAREOUS OOZE IN SURFACE SEDIMENTS
| Siliceous ooze | Calcareous ooze | |
|---|---|---|
| Surface water temperature | Cool | Warm |
| above sea floor deposits | ||
| Main location found | Sea floor beneath cool surface water in high latitudes | Sea floor beneath warm surface water in low latitudes |
| Other factors | Upwelling brings deep, cold, nutrient-rich water to the surface | Calcareous ooze dissolves below the CCD |
| Other locations found | Sea floor beneath areas of upwelling, including along the equator | Sea floor beneath warm surface water in low latitudes along the mid-ocean ridge |
Reference: All images and content are taken from Essentials of Oceanography by Alan P. Trujillo and Harold V. Thurman, 12th Edition.