What Resources Do Marine Sediments Provide?
The ocean floor harbors a wealth of potential mineral and organic assets. However, a substantial portion of these resources remains difficult to access, requiring advanced technological solutions and incurring elevated costs for extraction. Despite these obstacles, several of these prospects are among the most notable targets for marine exploration.
Energy Resources
Among the most significant energy assets linked to marine sediments are petroleum and gas hydrates.
PETROLEUM

The ancient remnants of microscopic marine organisms, preserved within ocean sediments before undergoing decomposition, serve as the primary source of present-day petroleum, encompassing both oil and natural gas deposits. More than 95% of the economic yield from nonliving oceanic extractions is derived from petroleum products, making it the most crucial of all marine-derived energy resources.
The proportion of global oil derived from offshore regions has risen substantially—from minimal levels in the 1930s to over 30% in contemporary times. This significant escalation is primarily attributed to ongoing technological innovations utilized by offshore drilling platforms. Major subsea oil reserves are situated in the Persian Gulf, the Gulf of Mexico, off the coast of Southern California, within the North Sea, and throughout the East Indies. It is also highly probable that additional reserves exist off northern Alaska, in the Canadian Arctic, beneath Asian seas, and along the coasts of Africa and Brazil. Given the extremely limited probability of discovering major new onshore reserves, future petroleum exploration will remain intensive in offshore regions, particularly within the deeper zones of the continental margins. Nonetheless, a critical limitation associated with offshore petroleum development is the inevitable occurrence of oil spills, which typically result from unintentional leaks or blowouts during the drilling phase.
GAS HYDRATES
Commonly referred to as clathrates (from clathri, meaning a lattice), gas hydrates are unusually compact crystalline compounds composed of water and natural gas. These unique structures develop exclusively under high-pressure conditions, where cold water and gas molecules are compressed into a solid resembling ice. While gas hydrates can trap various gases—including carbon dioxide, hydrogen sulfide, and more complex hydrocarbons such as ethane and propane—the most prevalent form occurring in the natural environment is methane hydrate. These notable deposits are found beneath Arctic permafrost regions on land and beneath the seafloor, where their presence was first documented in 1976.
Deep-ocean sediments, characterized by elevated pressure and low temperatures, offer an ideal setting for the formation of structures in which natural gas becomes encapsulated within a lattice-like framework of water molecules. Drilling expeditions into these gas hydrate zones have recovered core samples consisting of mud interspersed with fragments or layers of gas hydrate “ice,” which rapidly fizz and disintegrate when subjected to the warmer, low-pressure environment at the ocean’s surface. Although these hydrates bear a superficial resemblance to regular ice, they are flammable—igniting when exposed to flame due to the release of methane and other combustible gases during the vaporization process.
The majority of marine gas hydrates originate from the activity of bacteria that decompose organic material deposited in seafloor sediments, producing methane along with smaller quantities of ethane and propane. Under appropriate conditions of high pressure and low temperature, these gases are incorporated into the hydrate structure. While much of the ocean floor below depths of 525 meters (1720 feet) meets the fundamental criteria for hydrate formation, actual occurrences are generally limited to continental margins, where nutrient-rich surface waters promote the accumulation of organic-rich sediments on the seafloor.
Scientific investigations of the deep-ocean floor have identified at least 50 locations across the globe that may harbor extensive gas hydrate accumulations. Notably, methane seeps on the ocean floor sustain diverse biological communities, many comprising previously undocumented species.
The emission of methane from subsea sediments into the atmosphere can exert profound impacts on the global climate. Geological evidence indicates that, during certain periods in Earth’s history, fluctuations in sea level or seafloor instability have triggered the massive release of methane—recognized as the third-most-important greenhouse gas following water vapor and carbon dioxide. For example, sediment core analyses retrieved off the coast of Norway suggest that a sudden spike in global temperatures approximately 55 million years ago may have been caused by a violent discharge of gas hydrates from the seabed. At present, a significant concern is that modern climate change could sufficiently warm ocean waters, thereby activating the release of additional methane trapped beneath the ocean floor—potentially triggering further warming. Furthermore, abrupt gas hydrate discharges have been associated with submarine slope failures, which can generate seismic sea waves, or tsunami.
Some projections suggest that up to 20 quadrillion cubic meters (equivalent to 700 quadrillion cubic feet) of methane may be sequestered within marine sediments containing gas hydrates. This represents nearly twice the carbon content found in all of Earth’s coal, oil, and conventional natural gas reserves combined, positioning gas hydrates as a potentially fundamental source of global energy.
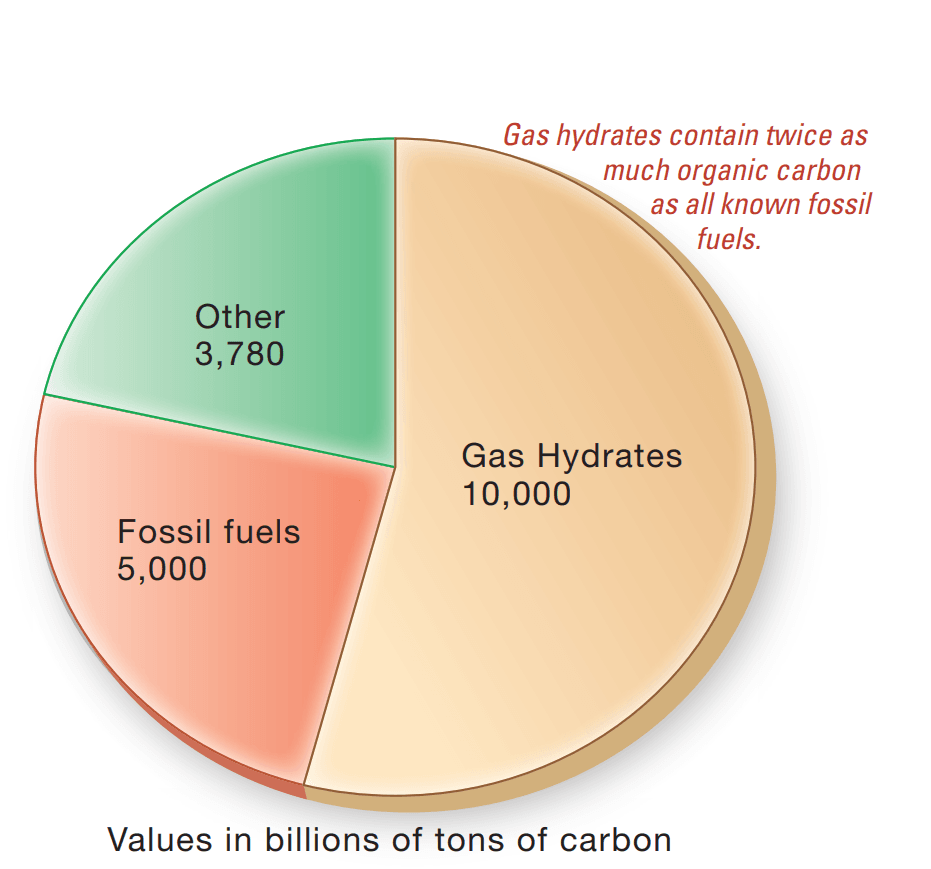
Despite their immense energy potential, gas hydrates present several critical limitations. A major drawback is their tendency to decompose rapidly under the temperature and pressure conditions found at the ocean surface. Moreover, gas hydrate deposits are generally too dispersed within the seafloor matrix to allow for cost-effective extraction. Another significant concern is the possibility of accidental methane emissions during commercial extraction, which could further intensify climate change linked to fossil fuel use. While ongoing technological innovations may address many of the specific challenges related to the safe and efficient recovery of methane from gas hydrates, there remain numerous scientific, engineering, and environmental issues that must be resolved before full-scale commercial production becomes viable. Nevertheless, a multinational research consortium is currently assessing the economic feasibility of extracting methane hydrates from the Nankai Trough off the coast of Japan, with the potential to initiate methane production as early as 2016.
Other Resources
Additional resources derived from marine sediments encompass sand and gravel, evaporative salts, phosphorite, manganese nodules and crusts, and rare-earth elements.
SAND AND GRAVEL
Comprising both rock fragments transported to the sea and shells of marine organisms, sand and gravel are extracted by offshore barges employing suction dredging techniques. These materials are primarily utilized as aggregate in concrete, as fill material for grading operations, and for beach nourishment on recreational shorelines. In terms of economic importance, offshore sand and gravel rank as the second-largest deposit on the sea floor, following only petroleum in value.
Offshore deposits serve as a major source of sand and gravel along the coasts of New England, New York, and throughout the Gulf Coast. Numerous European nations, as well as Iceland, Israel, and Lebanon, also rely heavily on these marine-derived materials.
Certain offshore sand and gravel deposits contain valuable minerals. For instance, gem-quality diamonds are extracted from gravel layers located on the continental shelf off the coasts of South Africa and Australia, where historical sea level fluctuations allowed wave action to rework these sediments. Additionally, tin-rich sediments have been commercially mined offshore in Southeast Asia, particularly from Thailand to Indonesia. Platinum and gold have been identified in deposits adjacent to coastal gold mining regions across the globe, and certain Florida beach sands possess high titanium content. The greatest unexplored potential for extracting metallic minerals from offshore sand deposits likely exists along the western coast of South America, where Andean-derived metals are transported seaward by river systems.
EVAPORATIVE SALTS
As seawater undergoes evaporation, the salt concentration increases until the dissolved ions can no longer remain in solution, resulting in the precipitation of salt deposits. Extensive seafloor salt layers suggest that entire marine basins, such as the Mediterranean Sea, may have experienced complete desiccation during certain periods of geologic history.

The most economically significant salts derived from marine sediments are gypsum and halite. Gypsum is a key component in plaster of Paris, which is utilized for producing casts and molds, and serves as the primary ingredient in gypsum board (also referred to as wallboard or sheet rock). Halite, commonly known as table salt, is extensively employed for seasoning, curing, and preserving food. Additionally, it is applied in road de-icing, water conditioning systems, agricultural practices, and the textile industry for fabric dyeing.
Beyond these applications, halite plays a crucial role in the synthesis of various chemical products, including sodium hydroxide (used in soap manufacturing), sodium hypochlorite (for disinfectants, bleaching agents, and PVC piping), sodium chlorate (used in herbicides, matches, and fireworks), and hydrochloric acid (applied in both chemical processing and pipe descaling). The production and utilization of salt represents one of the oldest and most fundamental sectors in the chemical industry.
PHOSPHORITE (PHOSPHATE MINERALS)
Phosphorite is a sedimentary rock composed of various phosphate minerals containing phosphorus, an essential nutrient for plant growth. As a result, phosphorite deposits are utilized in the production of phosphate fertilizers. Although commercial-scale marine phosphorite mining is not currently active, estimates indicate that the total marine reserve exceeds 45 billion metric tons (equivalent to 99 trillion pounds). Marine phosphorite is primarily located at depths less than 300 meters (1000 feet) along the continental shelf and slope, especially in areas characterized by upwelling and high biological productivity.
Certain shallow sand and mud deposits contain phosphate concentrations of up to 18%. Many phosphorite formations appear as nodules—solid masses with a hard crust encasing a central nucleus. These nodules may range in size from that of a sand grain to over 1 meter (3.3 feet) in diameter, and can possess phosphate contents exceeding 25%. By comparison, terrestrial sources of phosphate—such as those in Florida—typically contain over 31% phosphate due to groundwater leaching, and the region supplies nearly one-quarter of the global phosphate market.
MANGANESE NODULES AND CRUSTS
Manganese nodules are rounded, durable, metallic aggregates ranging in size from golf balls to tennis balls. These nodules contain substantial concentrations of manganese and iron, along with smaller but economically valuable amounts of copper, nickel, and cobalt—all of which have diverse industrial applications. During the 1960s, mining corporations initiated evaluations of the technical feasibility of harvesting manganese nodules from the deep-ocean floor. Surveys reveal that vast expanses of the seabed, particularly within the Pacific Ocean, are abundantly covered with these metallic formations.
From a technological perspective, extracting manganese nodules from the deep sea is entirely viable. However, the political complexities of assigning international mining rights in areas located far from national jurisdictions have significantly delayed the development of this resource. Furthermore, environmental implications surrounding deep-sea mining activities remain insufficiently explored, posing an additional obstacle to the commercial exploitation of manganese-rich deposits.
Scientific evidence indicates that the formation of manganese nodules requires at least several million years, depending on a specific combination of physical and chemical conditions that are likely transient in any given location. Consequently, manganese nodules are considered a nonrenewable resource, meaning that once extracted, they will not be naturally replenished within any foreseeable timeframe.
Among the five principal metals commonly present in manganese nodules, cobalt is the only one classified as strategic—a designation signifying its essential role in maintaining national security for the United States. Cobalt is necessary for producing dense, high-strength alloys used in high-speed cutting tools, high-performance permanent magnets, and critical components such as jet engine parts. At present, the United States is entirely dependent on imports of cobalt, primarily from extensive deposits located in southern Africa.
Nonetheless, the United States has evaluated deep-ocean nodules and crusts—which are hard mineral coatings on existing rocks—as a more reliable source of cobalt. During the 1980s, cobalt-enriched manganese crusts were discovered on the upper slopes of islands and seamounts located relatively nearshore and within the jurisdiction of the United States and its territories. The cobalt concentration within these crusts is approximately 1.5 times greater than that of the highest-grade African ores, and at least twice as rich as that found in deep-sea manganese nodules. Despite this notable resource potential, interest in extraction has diminished due to a decline in global metal prices, especially from land-based sources.
RARE-EARTH ELEMENTS
Rare-earth elements comprise a group of 17 chemically similar metallic elements, including lanthanum and neodymium, which are essential to a broad range of electronic, optical, magnetic, and catalytic technologies. These elements are employed in a wide array of modern devices, such as cell phones, television displays, fluorescent lighting, and batteries used in electric vehicles. In recent years, the demand for rare-earth elements has increased dramatically, with China currently supplying nearly 90% of the global market.
Over millions of years, deep-sea hydrothermal vents associated with mid-ocean ridges have extracted rare-earth elements from seawater, subsequently concentrating them in seafloor muds. A recent scientific investigation into rare-earth element distribution across the Pacific Ocean floor revealed that certain areas are especially enriched. For instance, one submarine region near Hawaii, covering just 1 square kilometer (0.4 square mile), contains up to 25,000 metric tons (55 million pounds) of rare-earth elements. In total, current estimates indicate that the ocean floor may contain greater quantities of rare-earth elements than all terrestrial deposits identified to date.
What are the primary energy resources found in marine sediments?
The most significant energy resources linked to marine sediments are petroleum (including oil and natural gas) and gas hydrates. Petroleum, formed from the ancient remnants of microscopic marine organisms, accounts for over 95% of the economic yield from nonliving oceanic extractions. Gas hydrates, or clathrates, are compact, ice-like crystalline compounds of water and natural gas, with methane hydrate being the most common form.
Reference: All images and content are taken from Essentials of Oceanography by Alan P. Trujillo and Harold V. Thurman, 12th Edition.