What are the stable isotopes of oxygen and their abundances?
There are three stable isotopes of oxygen which have the following abundances:
- 16O: 99.763%
- 17O: 0.0375%
- 18O: 0.1995%
The isotope ratio 18O/16O is typically measured in oxygen isotope studies, and δ values are calculated from this ratio. There are two main isotopic standards used for these measurements: PDB and SMOW. PDB refers to a belemnite from the Cretaceous Peedee formation in South Carolina, commonly used for carbon isotope measurements. SMOW (Standard Mean Ocean Water) was initially a theoretical water sample with oxygen and hydrogen isotope ratios matching those of standard ocean water. Today, a water standard known as Vienna-SMOW (V-SMOW) is used, distributed by the Atomic Energy Agency in Vienna. V-SMOW has an 18O/16O ratio identical to SMOW and a D/H ratio matching the original definition of SMOW.
How are δ18O values related between V-SMOW and PDB?
δ18OV-SMOW = 1.03091 × δ18OPDB + 30.01δ18OPDB = 0.97002 × δ18OV-SMOW - 29.98In addition to SMOW, the Standard Light Antarctic Precipitation (SLAP) is sometimes used, with a δ18O value of -55.5% relative to SMOW.
How is oxygen liberated for isotope analysis?
Oxygen is liberated from silicates and oxides through fluorination with F2 or BrF5, then reduced to CO2 at high temperatures for measurement in a mass spectrometer. For carbonates, carbon dioxide is liberated using >103% phosphoric acid. When determining oxygen isotope ratios in water, the sample is equilibrated with a small amount of CO2, and the oxygen isotope ratio in the CO2 is measured. The known water-CO2 fractionation factor allows calculation of the 18O/16O ratio in water, with a precision of δ18O values around 0.1-0.2%.
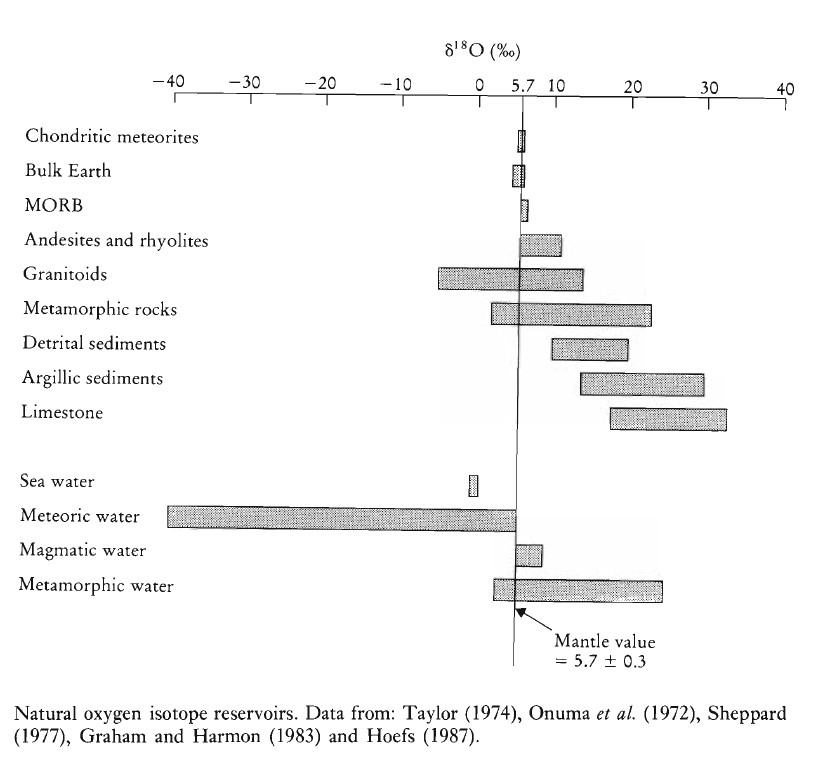
What are the variations of δ18O in nature?
δ18O values in nature vary by approximately 100%, with about half of this range found in meteoric water. Chondritic meteorites exhibit a very narrow range of δ18O values. The Earth’s mantle has a consistent δ18O value of 5.7 ± 0.3%, which has remained stable over time for both the Earth and the Moon. However, research by Kyser et al. (1982) found that alkali basalts in Hawaii are enriched in δ18O by 0.5 to 1.0% compared to tholeiites, suggesting distinct mantle sources. There is evidence of minor isotopic heterogeneities within the mantle.
Most granites, metamorphic rocks, and sediments are enriched in δ18O relative to the mantle, while seawater and meteoric waters are depleted, forming complementary δ18O reservoirs.