Proximate analysis
The most common analysis is proximate analysis. A proximate analysis is a common laboratory procedure to provide the fundamental composition of coal. It is used on coal during exploration, mining, and preparation. This analysis is also widely used for industrial purposes and for grading the coals.
Proximate analysis is a measure of the relative percentage of volatile and non-combustible mineral matter.
Proximate analysis of coal includes the determination of:
- Moisture
- Volatile matter
- Fixed carbon
- Ash content
Moisture:
Moisture in coal occurs in four possible focuses:
- Surface moisture: water held on films on the surface of coal particles.
- Hygroscopic moisture: water held under the capillaries of coal substances.
- Decomposition moisture: Water uncomposed in one of the coal organic compounds.
- Mineral moisture: water that focuses on part of the crystal silicates of clays and other minerals present in the coal.
Adventitious moisture
Sample collected from mines or stockpiles is usually wet with a variable amount of adventitious moisture from seepage, rain and other sources.
Inherent moisture
Like moisture in pores of the coal, which is drying by 100 degree Celsius, some mineral and decomposition moisture may not be liberated until temperature exceeds 500 degree Celsius.
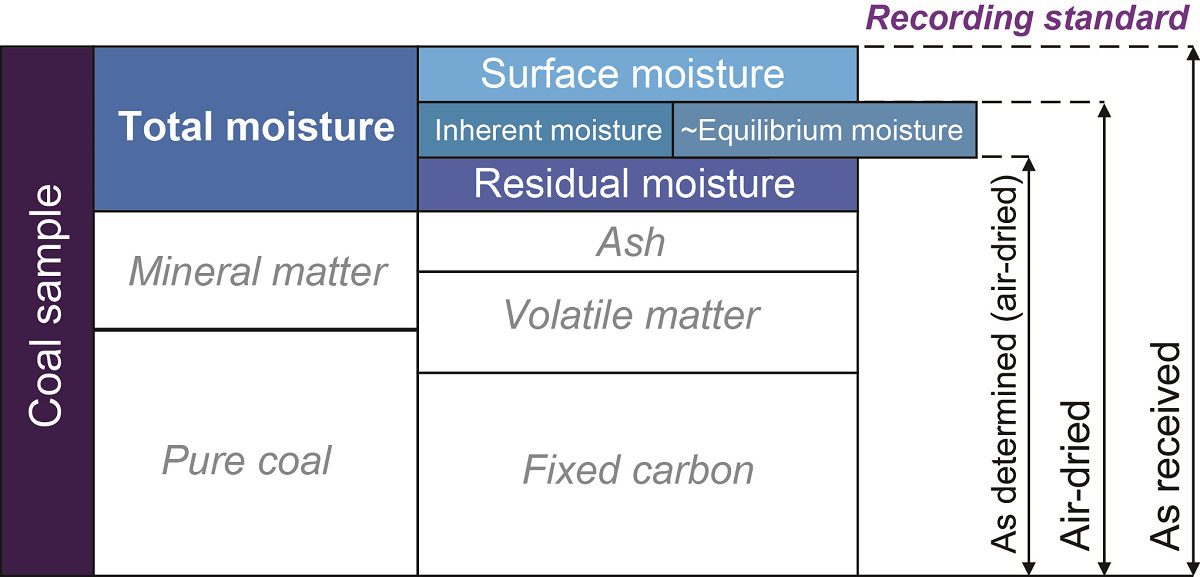
Total moisture:
Adventitious moisture + Inherent moisture
Methodology:
For determination of inherent moisture 1gm of air-fried coal (212 microns) is taken in a slice dish heated to a temperature of 108± 20C in an oven. The sample is cooled at room temperature and weighed again.
The less in weight before and after the heating is taken is moisture.
The moisture content of low-rank coal is greater than 20% whereas in the highest-rank coal anthracite moisture is less than 2%.
Sometimes free moisture is of added advantage, especially for fining boilers. For optimal thermal efficiency about 5% total moisture in coal is required
For making coke about 3 to 5 % of free moisture is essential for the best result.
M%= (weight loss/ Total weight of sample) X 100
Where M= Moisture
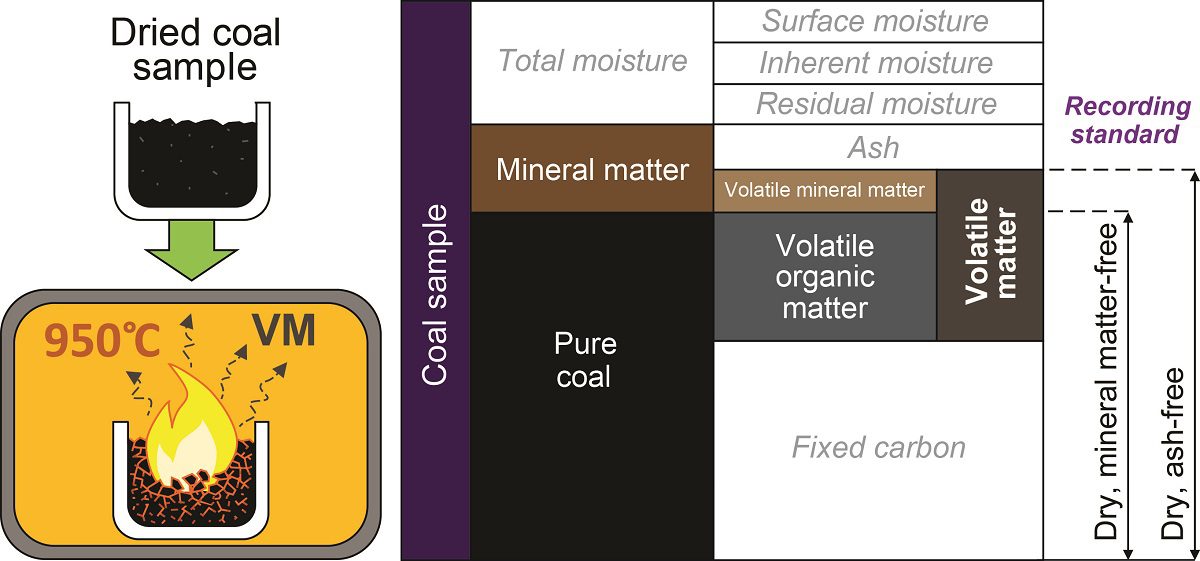
Measurement of volatile matter:
Volatile matters are methane hydrocarbons, hydrogen and carbon monoxide, and incombustible gases like carbon dioxide and nitrogen found in coal.
Volatile matter represents the component of the coal, except for moisture, that is liberated at high temperatures in the absence of air.
When coal is heated in the absence of air for 7 min at 9500 centigrade then the weight of the coal changes with respect to the initial weight of the coal is expressed as volatile content.
%Volatile matter = (Weight loss due to VM/ total weight of sample) X 100
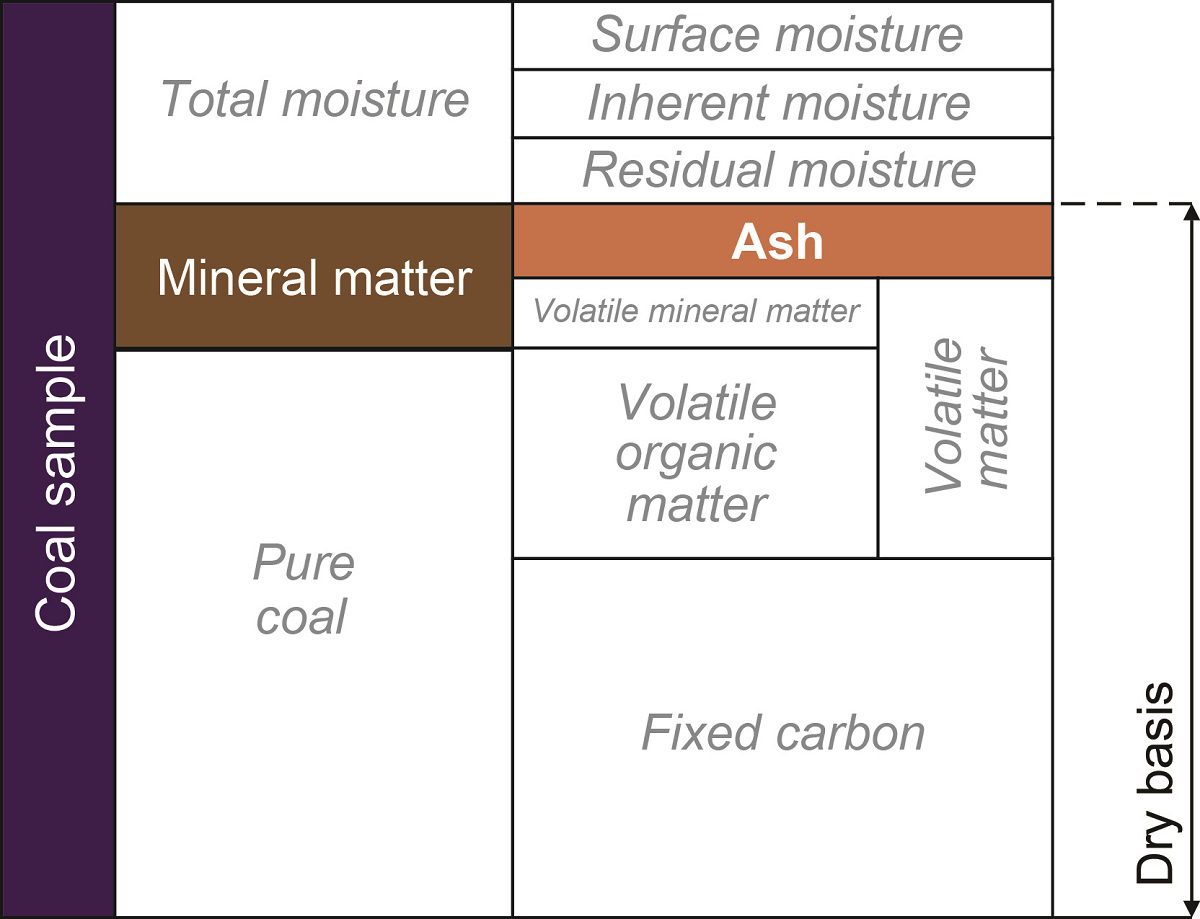
Ash content:
The non-combustible inorganic residue that remains out when coal is burned is ash of coal.
It represents bulk of mineral matter in the coals, after volatile components such as co2 (from carbonate), SO2 (from sulfide), and H2O (from clays) have been driven off from mineral compounds such as carbonates, sulfides, and clays.
Ash is the weight of residue obtained after complete combustion of 1g of coal at 700-750C.
Ash%= (weight of ash remaining/ weight of sample) X 100
Fixed carbon:
The fixed carbon of coal is the carbon found in the material that remains after the volatile matter has been expelled.
It represents the decomposition residue of coal. Organic components, carry with it small amounts of nitrogen, Sulphur, hydrogen, and possibly oxygen observed on chemically combined material.
The fixed carbon is not directly determined but is the difference in air-dried coal, between the sum of the other components (moisture, volatile matter, ash) and 100%
Fixed carbon%= 100-(M%+V.M%+A%)
Where, M= Moisture
V.M = Volatile matter
A= Ash
The fixed carbon content is used as an index of the yield of coke expected from coal on carbonization.
Basis of Analytical Data:
It is important to understand how the moisture, ash, volatile matter, and fixed carbon relate to one another, and to the basis that analytical data are presented.
-
‘As received’ basis (ar), also as sampled;
The data are expressed as percentages of the coal including the total moisture content, i.e. including both the surface and the air-dried moisture content of the coal.
-
‘Air-dried’ basis (adb):
The data are expressed as a percentage of air-dried coal; this includes the air-dried moisture but not the surface moisture of the coal.
-
‘Dry’ basis (dry):
The data are expressed as percentages of the coal after all the moisture has been removed.
-
‘Dry ash-free’ basis (daf):
The coal is considered to consist of volatile matter and fixed carbon on the basis of recalculation with moisture and ash removed.
-
Dmmf (dry Mineral matter-free basis):
The total amount of mineral rather than ash is determined, so that the volatile matter content is the mineral matter can be removed,
V.M (dry mineral matter free or dmmf basis) = [V.M(air dried)/ 100 – (M+ 1.A)] X 100
Ultimate Analysis of Coal:
The ultimate analysis of coal consists of the determination of carbon and hydrogen as gaseous of its complete combustion.
The calculation of the amounts of those elements that have a direct bearing on the usability of the coal is necessary. These may include forms of sulfur, chlorine, and phosphorus, an analysis of those elements making up the mineral matter content of the coal, and selected trace elements.
Carbon and hydrogen:
When coal is burnt in a current of oxygen. All the hydrogen is converted into water and all the carbon to caron dioxide. This carbon dioxide is liberated from carbonate minerals that may be present.
H2O is derived from water is clay or other hydrogen minerals. Hydrogen is also formed in the inherent moisture of the air-dried coal, and thus any measure of the total carbon and hydrogen includes a certain amount of material derived from inorganic source.
Determination:
It is based on heating the air-dried coal in a stream of dry oxygen, and collecting the CO2 and water produced in a series of absorption tubes.
C(daf)= [C (air-dried)/100-(M+A)] X 100
H(daf) = [H(air-dried)/100-(M+A)] X 100
Nitrogen:
The nitrogen found in coals appears to be mainly confined to organic compounds.
Some nitrogen compounds may occur in pore waters, especially in brown coal.
Determination:
It is determined by Kjeldahl method. Coal is heated with concentrated sulphuric acid containing potassium sulphate, in the presence of a catalyst, to convert the nitrogen into ammonium sulphate.
N(daf) = [N(air-dried)/100-(M+A)] X 100
Sulphur
Sulphur may occur in coal in several ways:
- Organic Sulphur-
- As Sulphide mineral
- As Sulphate mineral
The total Sulphur content of coal is determined gravimetrically by Eschka Method. In this method, a powdered coal sample is heated in intimate contact with Eschka mixture in an oxidizing atmosphere to remove combustible matter and to convert all forms of Sulphur to sulphate.
