What is meant by latent heat?
Water vapor exists as an invisible gas that only becomes perceptible when it undergoes transformation into larger liquid or solid (ice) particles. This alteration in physical form is recognized as a phase transition or change of state. The thermal energy necessary to induce such a transformation in a substance like water is known as latent heat.
But why is this specific heat termed “latent”?
To understand this, let us explore a phenomenon commonly encountered—the cooling effect produced by water evaporation.
Consider a microscopic analysis of a tiny droplet of distilled water. At the droplet’s surface, molecular escape or evaporation is continuously occurring. The molecules with the highest kinetic energy—those moving most rapidly—are the first to break free from the surface. As these faster-moving molecules depart, the average kinetic energy of the remaining molecules diminishes. Given that temperature is defined as a measure of average molecular motion, this reduction in kinetic energy reflects a lower temperature of the water.
Hence, evaporation serves as a cooling mechanism. The thermal energy required to facilitate the transition from liquid to gas may be derived from the water itself or from surrounding sources, such as the ambient air.
In routine life, we often perceive this evaporative cooling effect when emerging from a shower or swimming pool into a dry atmosphere. As a portion of the energy used to convert water on the skin into vapor is extracted directly from our body, a noticeable decrease in skin temperature may occur—frequently leading to the development of goose bumps. On particularly hot, arid, and windy days, such as those experienced in Tucson, Arizona, the cooling can become so intense that one may begin to shiver, even when the surrounding air temperature approaches approximately 38°C (100°F).
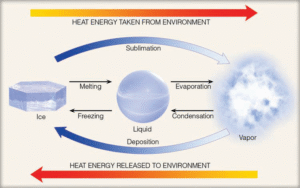
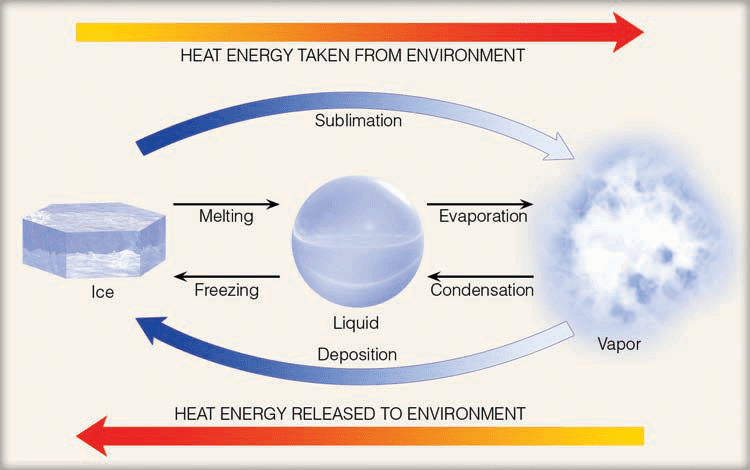
The thermal energy dissipated by liquid water during the process of evaporation can be conceptualized as being transferred to and retained within the water vapor molecules. This energy assumes a “stored” or “concealed” state, which is why it is referred to as latent heat. The term “latent” denotes that this energy remains undetectable, as the temperature of the transitioning molecule—from liquid to vapor—does not exhibit any perceptible change. However, this concealed thermal energy re-emerges as sensible heat—the kind of heat that can be felt, perceived, and quantified using a thermometer—once the vapor condenses back into liquid form. Accordingly, condensation, which is the reverse of evaporation, constitutes a warming phenomenon.
The thermal energy liberated when water vapor undergoes condensation to form liquid droplets is termed the latent heat of condensation. In contrast, the energy absorbed to convert liquid water into vapor, while maintaining the same temperature, is designated as the latent heat of evaporation or vaporization. Approximately 600 calories (2500 joules) are essential to evaporate one gram of water at ambient room temperature. Given that several hundred grams of water may evaporate from the human body, it is unsurprising that individuals often experience a cooling sensation immediately after a shower, before the skin has dried completely.
In a similar context, latent heat plays a notable role in maintaining the lower temperature of a beverage containing ice compared to one without it. As the ice melts, there is no change in its temperature. This occurs because the heat absorbed during the melting process merely serves to disrupt the rigid crystalline lattice of the ice, thereby transitioning it into liquid form without influencing its thermal state. The energy employed in facilitating this transformation is known as the latent heat of fusion (or melting), with roughly 80 calories (335 joules) required to melt a single gram of ice. Consequently, the heat introduced into a chilled beverage containing ice is primarily consumed in melting the ice, whereas the same amount of heat added to a drink without ice directly increases the liquid’s temperature.
Specific Heat of Various Substances
| SUBSTANCE | SPECIFIC HEAT Cal/(g × °C) | J/(kg × °C) |
|---|---|---|
| Water (pure) | 1.00 | 4186 |
| Wet mud | 0.60 | 2512 |
| Ice (0°C) | 0.50 | 2093 |
| Sandy clay | 0.33 | 1381 |
| Dry air (sea level) | 0.24 | 1005 |
| Quartz sand | 0.19 | 795 |
| Granite | 0.19 | 794 |
If one gram of water at 0°C reverts to ice at the same temperature, an equivalent amount of energy—80 calories—is released as sensible heat to the surrounding environment. Hence, during melting, heat is absorbed, whereas during freezing, heat is emitted.
The thermal energy essential for converting ice directly into vapor—a transformation identified as sublimation—is termed the latent heat of sublimation. To fully convert one gram of ice into vapor at 0°C, approximately 680 calories are necessary. This total comprises 80 calories for the latent heat of fusion and an additional 600 calories for the latent heat of evaporation. If this vapor subsequently reverts to the solid phase—through a process known as deposition—an equivalent amount of energy, roughly 680 calories (2850 joules), is emitted into the surrounding environment.
When a substance changes its state from solid to liquid to gas (i.e., from left to right in the transformation sequence), thermal energy is absorbed by the substance and removed from the environment. As a result, melting, evaporation, and sublimation induce cooling in the immediate surroundings. Conversely, when the transformation proceeds in the opposite direction—from gas to liquid to solid—the substance releases energy, which is transferred to the environment. Therefore, freezing, condensation, and deposition are all warming processes for their surroundings.
Latent heat serves as a crucial source of atmospheric energy. Once water vapor molecules have separated from the Earth’s surface, they are carried aloft by the wind, much like how dust is swept by a broom. As they ascend into higher atmospheric layers—where temperatures are significantly lower—these vapor molecules undergo condensation and freezing, transforming into liquid and ice cloud particles. These phase changes release immense quantities of thermal energy into the environment. This released heat becomes a vital driver for various meteorological phenomena, including hurricanes, mid-latitude cyclones, and thunderstorms.
When water vapor, initially evaporated from warm, tropical oceans, is transported to polar regions, it condenses and discharges its stored heat energy. This integrated cycle of evaporation, atmospheric transport, and condensation represents a fundamental mechanism for redistributing thermal energy (and water) within the Earth’s atmosphere.