As an oceanographer who has spent decades sampling the vast waters of our planet, from the frigid poles to the balmy tropics, I can tell you that the ocean is far more than just water. It is a complex, life-sustaining chemical soup, a dilute solution of nearly every known element. The most defining characteristic of this solution is its saltiness, or salinity. Understanding salinity is the first step to understanding the physical, chemical, and biological engine that is our global ocean.
What Exactly Is Ocean Salinity?
At its simplest, salinity is the measure of all the dissolved salts in water. You might be familiar with the taste of salt in seawater—that’s primarily sodium chloride (NaCl), or common table salt. But that’s only part of the story.
The formal scientific definition, established to ensure consistency among researchers worldwide, is quite technical: Salinity is the total amount of solid material in grams contained in one kilogram of seawater, after all carbonate has been converted to oxide, all bromine and iodine replaced by chlorine, and all organic matter has been completely oxidized.
Let’s break that down: This definition is less about a simple measurement and more about creating a standardized, repeatable recipe. It ensures that when a scientist in England and a scientist in Japan measure a sample, they are measuring the exact same properties.
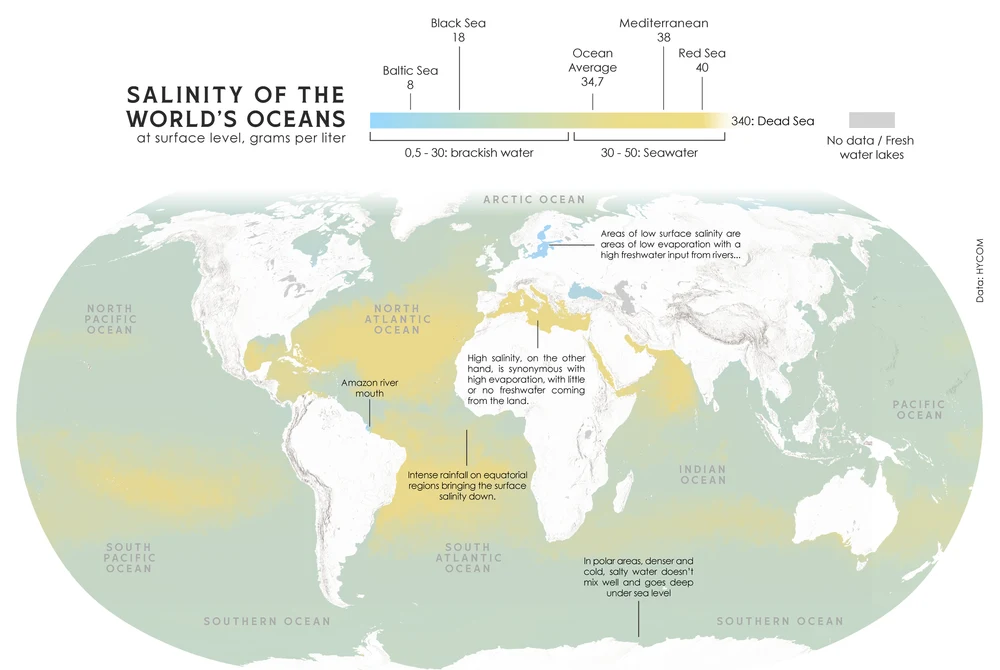
In practical terms, we express salinity in parts per thousand (‰) or practical salinity units (psu). The global average ocean salinity is about 35‰. This means that if you evaporated 1,000 grams (1 kilogram) of typical seawater, you would be left with 35 grams of dissolved salts.
The Ocean’s Elements
While the total amount of salt can vary from place to place, a remarkable discovery in oceanography is the Principle of Constant Proportions. This principle states that no matter the salinity, the relative proportions of the major dissolved ions remain virtually constant everywhere in the open ocean.
Think of it as a planetary recipe. Whether you have a small cup or a giant cauldron of this “ocean soup,” the ratio of ingredients is always the same. Water (H₂O) makes up about 96.5% of the ocean’s mass, while dissolved salts account for the other 3.5%.
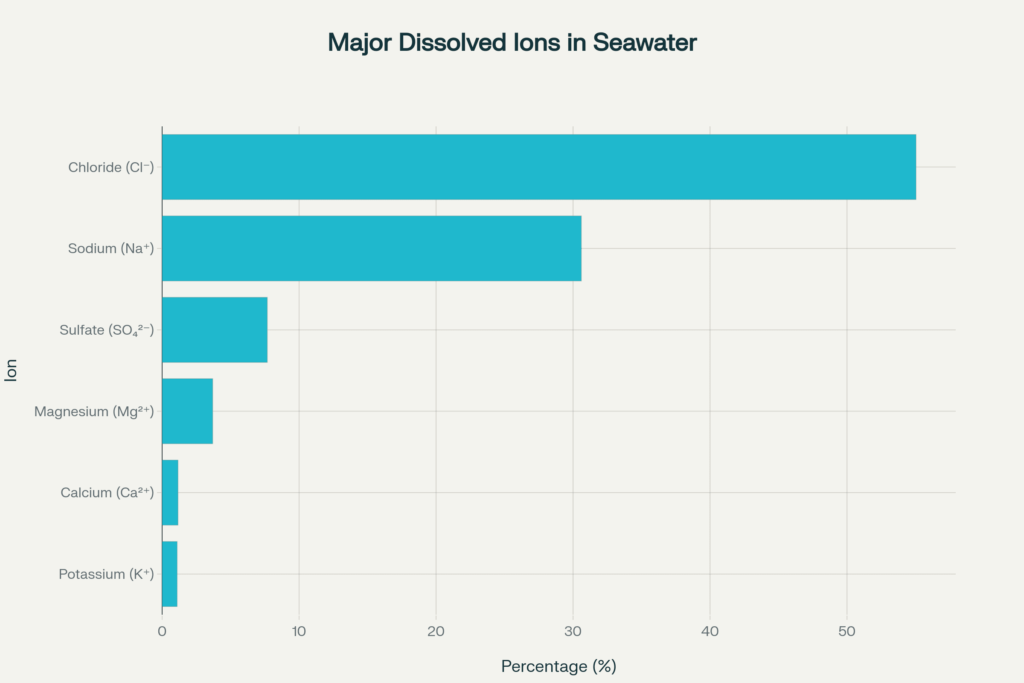
Of those salts, just six major ions make up over 99% of the dissolved solids:
| Ion | Chemical Symbol | Percentage of Total Salts |
|---|---|---|
| Chloride | Cl⁻ | 55.04% |
| Sodium | Na⁺ | 30.61% |
| Sulfate | SO₄²⁻ | 7.68% |
| Magnesium | Mg²⁺ | 3.69% |
| Calcium | Ca²⁺ | 1.16% |
| Potassium | K⁺ | 1.10% |
| Total | ~99.28% |
Beyond these are minor constituents (like bromine and carbon) and trace elements (like iron, zinc, and lithium) that, while small in quantity, are often vital for marine life.
Where Does the Salt Come From?
The ocean’s saltiness is the result of a geological-scale process that has unfolded over millions of years. The primary sources include:
- Weathering of Rocks on Land: Rainwater, which is slightly acidic, erodes rocks. This process releases mineral salts in their ionic form, which are then carried by rivers and streams into the ocean.
- Hydrothermal Vents: Water seeps into cracks in the seafloor, gets heated by magma, and dissolves minerals from the crust. This superheated water then erupts from hydrothermal vents, carrying a rich payload of dissolved minerals directly into the ocean.
- Volcanic Eruptions: Submarine volcanoes erupt, spewing gases and minerals into the water column.

Measuring Salinity: From Titration to Satellites
Our ability to measure salinity has evolved dramatically, reflecting our growing technological prowess.
- Historical Method (Chlorinity Titration): Because of the Principle of Constant Proportions, we know that Chloride ions make up about 55% of all dissolved solids. For decades, oceanographers could determine total salinity by meticulously measuring the concentration of chloride ions (chlorinity) in a sample through a chemical titration process. The relationship is precise: Salinity (‰) = 1.80655 × Chlorinity (‰) This method was accurate but incredibly time-consuming. For years, the global standard for this was maintained by labs in Copenhagen and later Wormley, England, which would distribute “Standard Seawater” ampules for calibrating instruments worldwide.
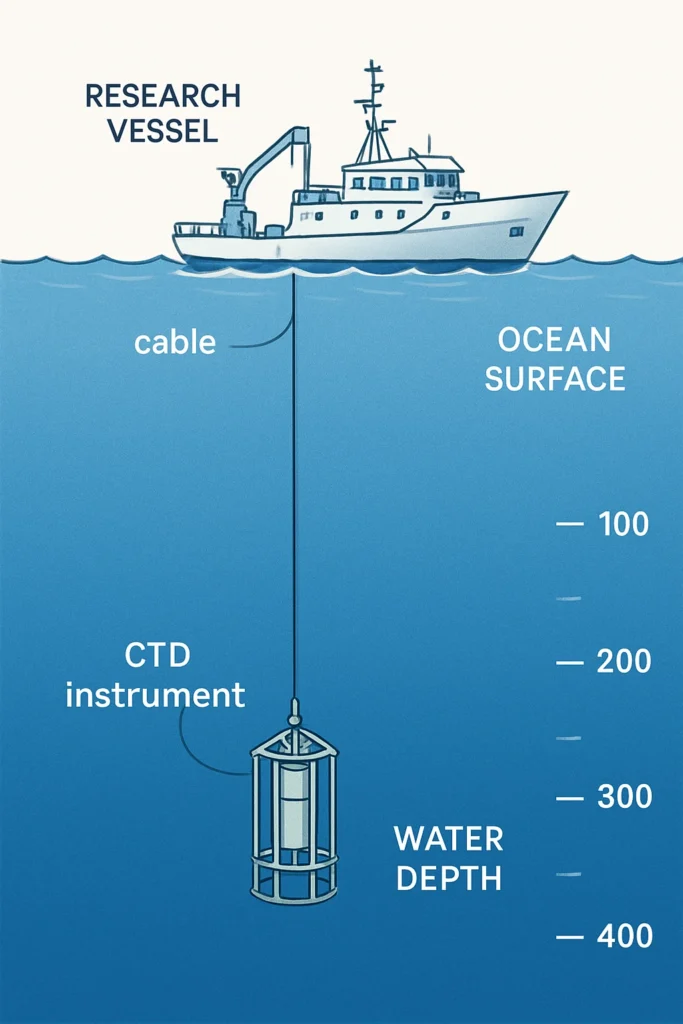
- The Modern Standard (The CTD): Today, the workhorse of physical oceanography is the CTD, which stands for Conductivity, Temperature, and Depth. This electronic instrument measures salinity based on a simple physical principle: the saltier the water, the better it conducts electricity. The CTD can be lowered from a ship, providing a continuous, high-resolution profile of the water column in real-time. This has revolutionized our ability to map ocean properties.
- Satellite Oceanography: In a groundbreaking leap, projects like NASA’s Aquarius mission and the ESA’s SMOS mission have allowed us to measure sea surface salinity from space, giving us a global, dynamic view of how salinity patterns change with the seasons and climate cycles.

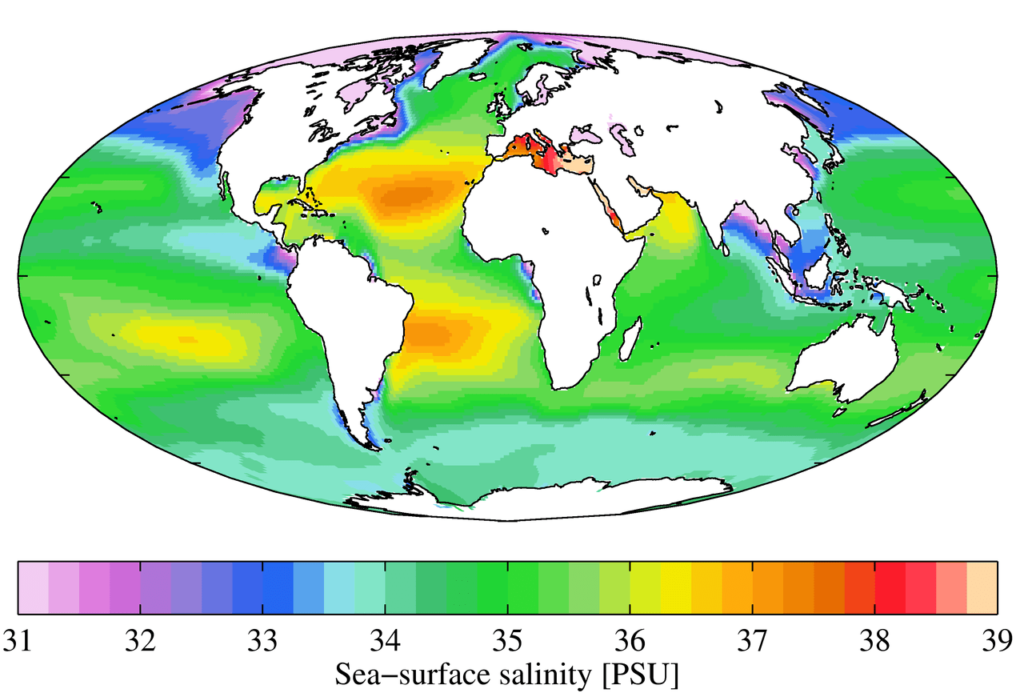
Why Salinity Varies Across the Globe
While the average salinity is 35‰, it is not uniform. Salinity is a function of the local balance between freshwater entering and leaving the ocean.
- Higher Salinity: Occurs in regions where evaporation is high, leaving the salt behind. The subtropical zones, like the “horse latitudes,” and enclosed seas in hot climates like the Red Sea and the Mediterranean Sea, have some of the highest salinities.
- Lower Salinity: Occurs where freshwater input is high. This includes:
- River mouths: The Amazon River, for example, creates a massive plume of freshwater that significantly lowers salinity for hundreds of kilometers into the Atlantic.
- Equatorial regions: High rainfall dilutes the surface waters.
- Polar regions: The melting of sea ice and glaciers releases vast amounts of freshwater.
Furthermore, when sea ice forms, it expels most of the salt, making the surrounding water even saltier and denser. This process is a key driver of deep-ocean circulation.

Why Salinity Is Crucial for Our Planet
Salinity is not just a scientific curiosity; it is a fundamental driver of Earth’s systems.
- Drives Ocean Circulation: Along with temperature, salinity determines the density of seawater. Cold, salty water is very dense and sinks, while warm, less salty water is lighter and stays at the surface. This density difference is the engine behind the Global Ocean Conveyor Belt (Thermohaline Circulation), a massive circulatory system that transports heat around the planet and regulates our climate.
- Governs Marine Life: Marine organisms are adapted to live within specific salinity ranges. Their cells must maintain a delicate water balance through osmoregulation. Estuaries, where freshwater rivers meet the salty ocean, are unique ecosystems that host specially adapted species capable of tolerating wide swings in salinity.
- Records Earth’s History: Programs like the Geochemical Ocean Sections (GEOSECS) have created a global inventory of ocean chemistry. By analyzing the chemical makeup of water masses, we can trace their origins, understand circulation patterns, and even study the biological cycles that shape marine ecosystems.
In conclusion, the salt in the ocean is a powerful force. It tells a story of geological time, drives our planet’s climate, and sets the boundaries for life in the vast marine world. Every drop of seawater carries this chemical signature, connecting the deepest abyss to the distant continents.
Reference: All images and content are taken from Essentials of Oceanography by Alan P. Trujillo and Harold V. Thurman, 12th Edition.